Cryotherapy is the most common treatment for actinic keratosis, but its effect is limited to individual lesions. Several topical drugs, however, are available that, in addition to treating individual actinic keratoses, target field cancerization and thereby act on subclinical lesions. Examples are 5-fluorouracil, imiquimod, diclofenac, and ingenol mebutate. We report on 17 patients with actinic keratoses treated with ingenol mebutate and describe our findings on treatment effectiveness, adherence, and tolerance. Complete and partial response rates were 35% and 53%, respectively. Ninety-four percent of patients fully adhered to treatment and 18% developed severe local reactions. Ingenol mebutate is an effective treatment for actinic keratosis. Although it has a similar rate of local reactions to other treatments available for actinic keratosis, its short treatment regimen favors better adherence.
La crioterapia es el tratamiento más frecuentemente utilizado para las queratosis actínicas, ejerciendo su efecto únicamente sobre lesiones individuales. Existen fármacos tópicos que tratan además el campo de cancerización, actuando sobre queratosis actínicas no clínicamente evidentes, entre los que se encuentran el 5-fluorouracilo, el imiquimod, el diclofenaco o el ingenol mebutato.
Presentamos 17 pacientes con queratosis actínicas tratados con ingenol mebutato y describimos las observaciones en relación con la efectividad, el cumplimiento terapéutico y la tolerancia del fármaco. Las tasas de respuesta completa y parcial fueron del 35% y del 53%, respectivamente. El cumplimiento fue correcto en un 94% de los casos. En el 18% de los pacientes existieron reacciones locales intensas.
El ingenol mebutato es efectivo para el tratamiento de las queratosis actínicas. Aunque presenta similar tasa de reacciones locales a los restantes tratamientos disponibles para esta indicación, su pauta corta de administración favorece el cumplimiento.
Actinic keratoses (AK) are considered by most authors to be premalignant lesions that can progress to invasive squamous cell carcinoma.1 In addition to lesion-directed therapies such as cryotherapy, the available treatment options for AK also include topical treatments that act on both the isolated—clinically visible—lesions and on the surrounding area of skin with chronic actinic damage and subclinical AK. Treatments include 5-fluorouracil, imiquimod (IMQ), diclofenac (DCF), and photodynamic therapy.1,2
Ingenol mebutate is a treatment indicated for AK that has only recently become available. It has been shown to be effective in the treatment of both individual lesions and the cancerization field, with treatment cycles of 3 consecutive days for the face and scalp and 2-day cycles for the trunk or limbs, achieving a complete cure rate of around 40%.1 The main difference between ingenol mebutate and other field cancerization therapies is that the treatment cycle is shorter and adherence to the dosing schedule is easier.
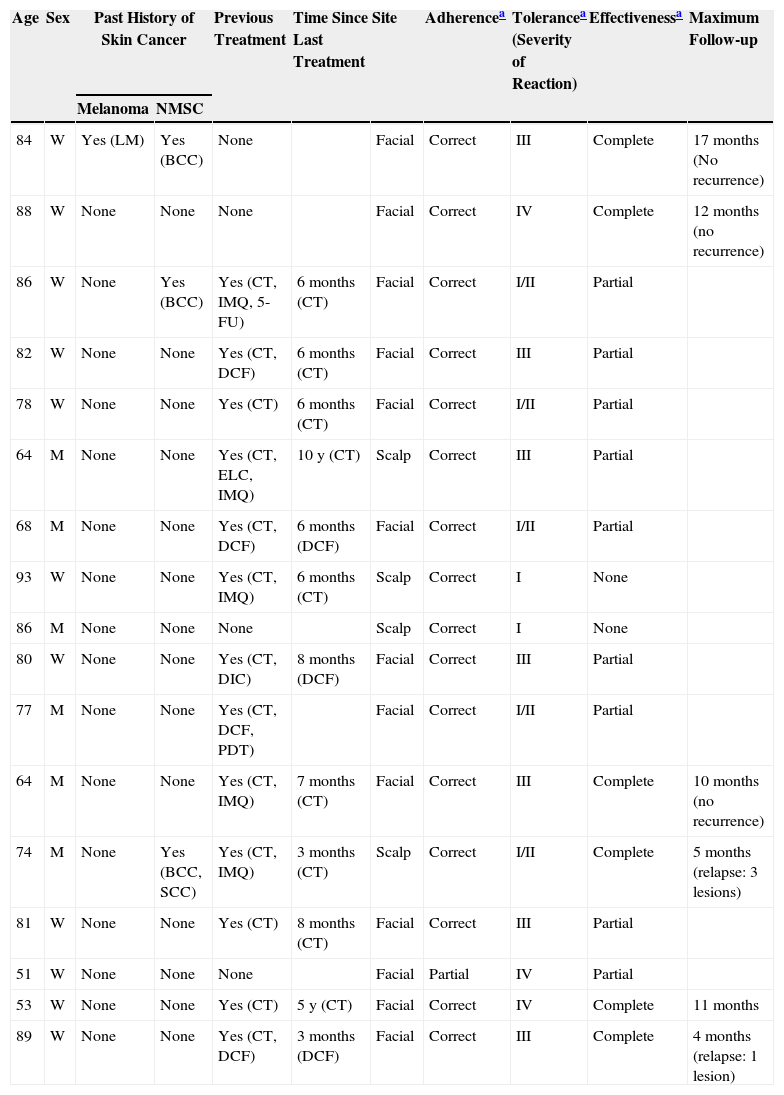
Case DescriptionsWe present a series of 17 patients treated with ingenol mebutate. Demographic and clinical characteristics were evaluated as well as treatment-related data (effectiveness, adherence to treatment, and tolerance) (Table 1). Effectiveness was evaluated at 2 months2 and was classified as follows: complete response if no abnormalities were detected in the treated area (normal skin); partial response if the abnormalities had diminished but were still present to some degree, making a cycle of any other treatment necessary; and no response if the lesions were unchanged. The regimen prescribed was 3 doses for facial lesions and 2 doses if the site affected was on the body. Correct adherence to treatment was defined as the application by the patient of all the doses prescribed; if any dose was skipped, adherence was defined as partial. To assess tolerance, the patients were asked about localized irritation at the site of application using the terminology specified in the Summary of Product Characteristics (minimum, type I; mild, type II; moderate, type III; and severe, type IV).
Patients Treated With Ingenol Mebutate.
| Age | Sex | Past History of Skin Cancer | Previous Treatment | Time Since Last Treatment | Site | Adherencea | Tolerancea (Severity of Reaction) | Effectivenessa | Maximum Follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|
| Melanoma | NMSC | |||||||||
| 84 | W | Yes (LM) | Yes (BCC) | None | Facial | Correct | III | Complete | 17 months (No recurrence) | |
| 88 | W | None | None | None | Facial | Correct | IV | Complete | 12 months (no recurrence) | |
| 86 | W | None | Yes (BCC) | Yes (CT, IMQ, 5-FU) | 6 months (CT) | Facial | Correct | I/II | Partial | |
| 82 | W | None | None | Yes (CT, DCF) | 6 months (CT) | Facial | Correct | III | Partial | |
| 78 | W | None | None | Yes (CT) | 6 months (CT) | Facial | Correct | I/II | Partial | |
| 64 | M | None | None | Yes (CT, ELC, IMQ) | 10 y (CT) | Scalp | Correct | III | Partial | |
| 68 | M | None | None | Yes (CT, DCF) | 6 months (DCF) | Facial | Correct | I/II | Partial | |
| 93 | W | None | None | Yes (CT, IMQ) | 6 months (CT) | Scalp | Correct | I | None | |
| 86 | M | None | None | None | Scalp | Correct | I | None | ||
| 80 | W | None | None | Yes (CT, DIC) | 8 months (DCF) | Facial | Correct | III | Partial | |
| 77 | M | None | None | Yes (CT, DCF, PDT) | Facial | Correct | I/II | Partial | ||
| 64 | M | None | None | Yes (CT, IMQ) | 7 months (CT) | Facial | Correct | III | Complete | 10 months (no recurrence) |
| 74 | M | None | Yes (BCC, SCC) | Yes (CT, IMQ) | 3 months (CT) | Scalp | Correct | I/II | Complete | 5 months (relapse: 3 lesions) |
| 81 | W | None | None | Yes (CT) | 8 months (CT) | Facial | Correct | III | Partial | |
| 51 | W | None | None | None | Facial | Partial | IV | Partial | ||
| 53 | W | None | None | Yes (CT) | 5 y (CT) | Facial | Correct | IV | Complete | 11 months |
| 89 | W | None | None | Yes (CT, DCF) | 3 months (DCF) | Facial | Correct | III | Complete | 4 months (relapse: 1 lesion) |
Abbreviations: 5-FU, 5-fluorouracil; BCC, basal cell carcinoma; CT, Cryotherapy; DCF, diclofenac; ELC, electrocoagulation; IMQ, imiquimod; LM, lentigo maligna; M, Man; NMSC, nonmelanoma skin cancer; PDT, photodynamic therapy; SCC, squamous cell carcinoma; W, woman.
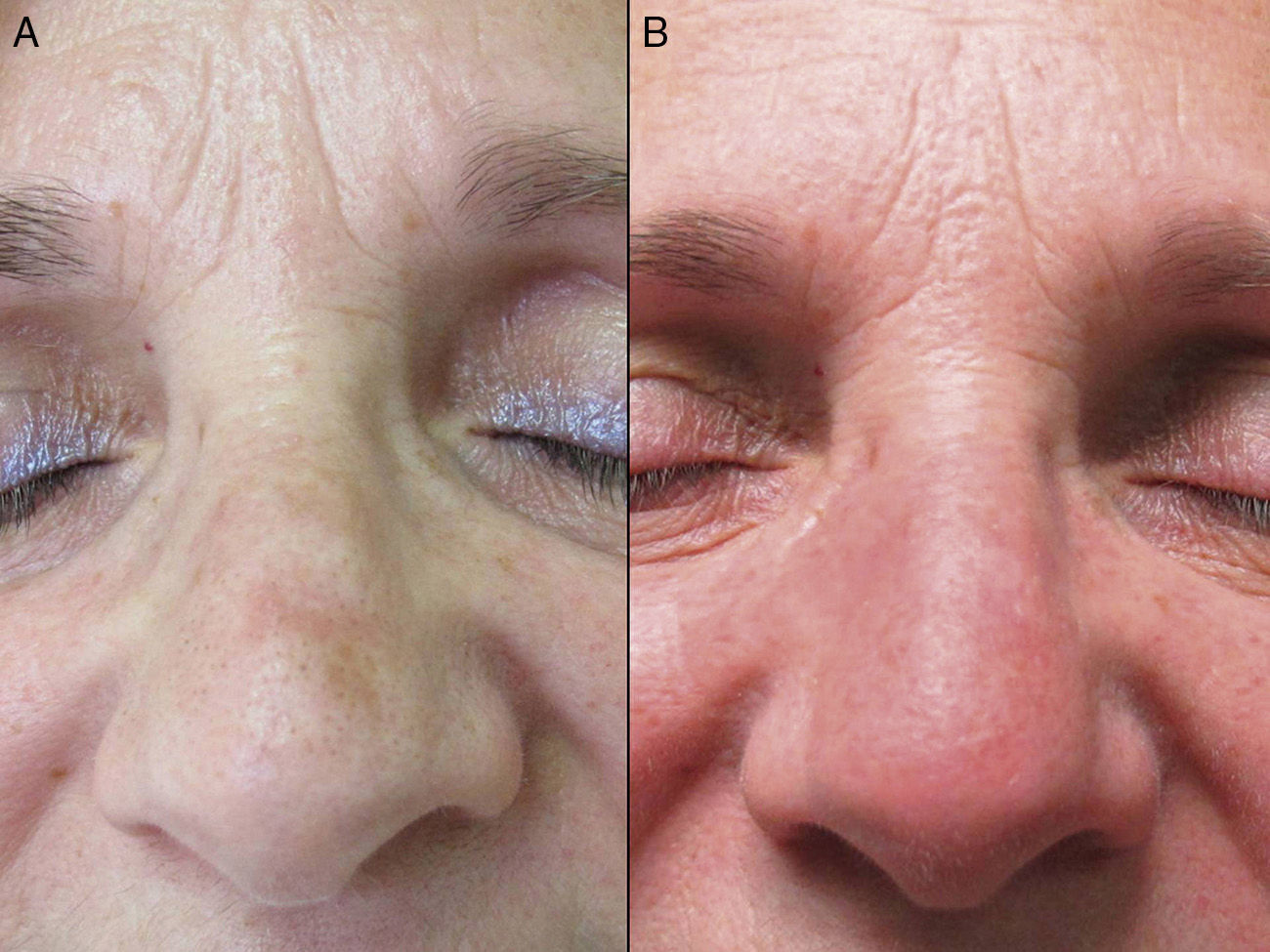
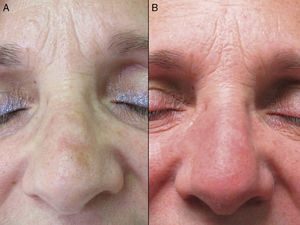
The mean age of the group of patients treated was 76 years. Only 1 patient had a history of melanoma skin cancer, and 3 patients a history of non-melanoma skin cancer. None of the prior skin cancer lesions were located in the area treated with ingenol mebutate. Fourteen of the 17 patients (82%) had previously received treatment for AKs, mainly cryotherapy (76%), imiquimod (29%), or diclofenac (29%). The mean interval between the last prior treatment and application of ingenol mebutate was 20 months (median, 6 months). All the patients in the present study received treatment with ingenol mebutate for AK lesions on the face (76%; 13/17) or scalp (24%; 4/17). The response to treatment was complete in 35% (6/17) and partial in 53% (9/17). In 2 cases (12%), there was no response. Figure 1 shows a case of complete response to ingenol mebutate. Long-term follow-up was variable after the assessment of response at 2 months post-treatment. Table 1 shows the maximum follow-up period and the clinical outcome at the end of follow-up for the patients who presented a complete response (mean: 10 mo, median: 11 mo; complete remission in 67% of cases). Adherence was correct in 94% (16/17) of the patients. The only case in which there was partial compliance was due to a type IV local reaction after application of the first dose; the following 2 applications were not administered because of the severe symptoms associated with that reaction (Fig. 2). In addition to that case, 2 other patients had local type IV reactions (3/17 in total, 18%). The rates for type III and type I/II reactions were similar: 41% in both groups. No relationship was observed between the severity of the reaction and a better or worse response (P=0.4; Fisher test). None of the patients reported cosmetic alterations or scarring in the treated area.
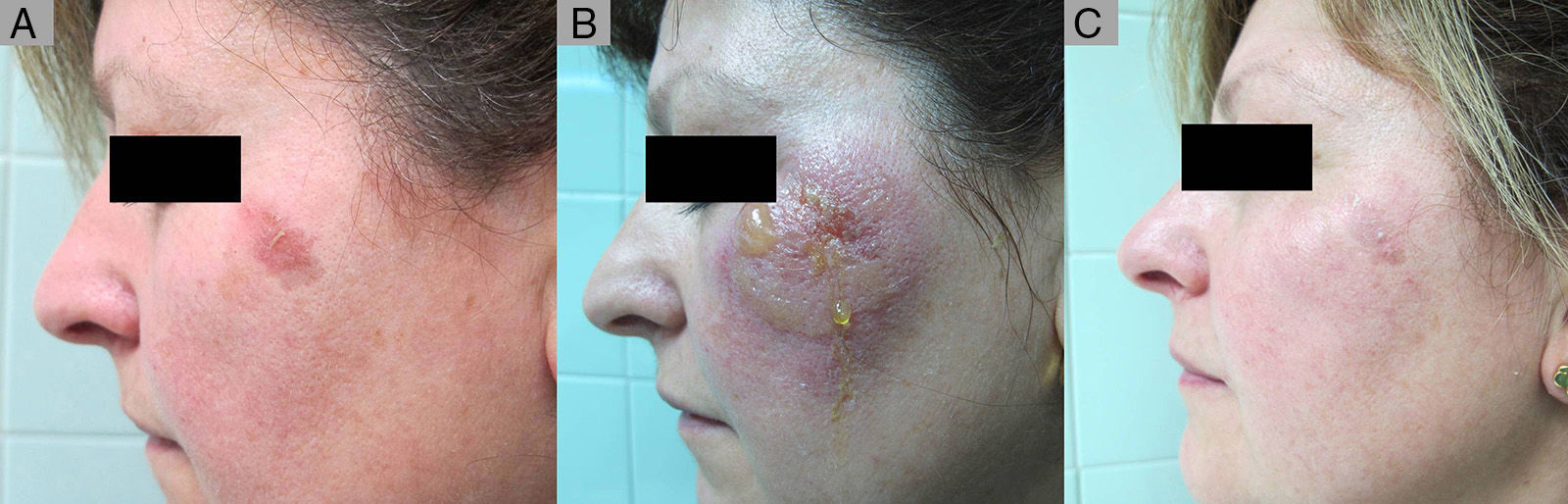
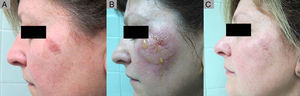
Partial Response to ingenol mebutate following treatment that was not completed due to a type iv local reaction. A, Non-hyperkeratotic actinic keratosis on the left malar. B, Type iv local reaction after administration of the first single dose of ingenol mebutate; the following 2 applications were not administered. C, Residual actinic keratosis. The patient required an additional session of cryotherapy.
In this case series of patients treated with ingenol mebutate in routine clinical practice, we observed a response rate of 88% (complete response, 35%; partial response, 53%), high adherence (94%), and frequent local skin reactions (minimal to mild, 41%; moderate to severe, 59%).
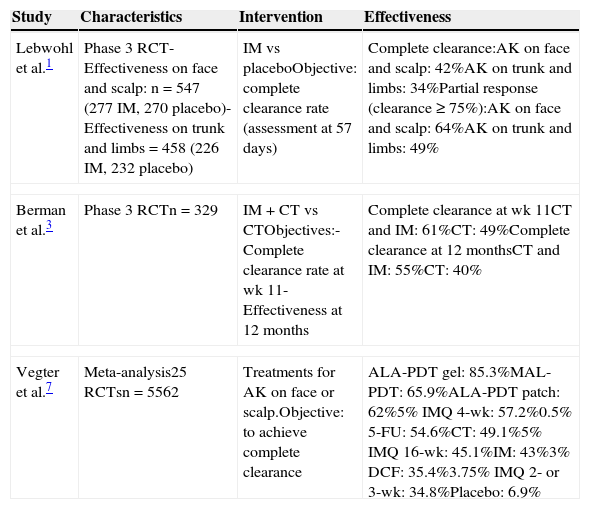
The response rates in this study were slightly lower than those reported in the literature (Table 2).1 It has been observed that the success rate may be higher when ingenol mebutate is combined with other therapies (Table 2).3
Main Studies That Have Assessed Effectiveness, Adherence, and Localized Reactions in Actinic Keratosis.
| Study | Characteristics | Intervention | Effectiveness |
|---|---|---|---|
| Lebwohl et al.1 | Phase 3 RCT- Effectiveness on face and scalp: n=547 (277 IM, 270 placebo)-Effectiveness on trunk and limbs=458 (226 IM, 232 placebo) | IM vs placeboObjective: complete clearance rate (assessment at 57 days) | Complete clearance:AK on face and scalp: 42%AK on trunk and limbs: 34%Partial response (clearance ≥ 75%):AK on face and scalp: 64%AK on trunk and limbs: 49% |
| Berman et al.3 | Phase 3 RCTn=329 | IM+CT vs CTObjectives:- Complete clearance rate at wk 11- Effectiveness at 12 months | Complete clearance at wk 11CT and IM: 61%CT: 49%Complete clearance at 12 monthsCT and IM: 55%CT: 40% |
| Vegter et al.7 | Meta-analysis25 RCTsn=5562 | Treatments for AK on face or scalp.Objective: to achieve complete clearance | ALA-PDT gel: 85.3%MAL-PDT: 65.9%ALA-PDT patch: 62%5% IMQ 4-wk: 57.2%0.5% 5-FU: 54.6%CT: 49.1%5% IMQ 16-wk: 45.1%IM: 43%3% DCF: 35.4%3.75% IMQ 2- or 3-wk: 34.8%Placebo: 6.9% |
| Study | Characteristics | Intervention | Effectiveness |
|---|---|---|---|
| Gupta and Paquet6 (Cochrane review update) | Meta-analysis of36 RCTsn=6473Data Collection: up till April 2012 | Treatments for AKObjective: to achieve complete clearance | No clearance percentage specified. Treatments are shown in order of effectiveness |
| All Sites | Scalp Only | Overall |
|---|---|---|
| 5% 5-FU >0.5%5-FU, ALA-PDT >5% IMQ>IM>MAL PDT>CT>DCF>placebo | 5% 5-FU>0.5%5-FU>IM>ALA-PDT>5%IMQ>MAL-PDT>CT>placebo | 5-FU>ALA-PDT≈5%IMQ ≈ IM≈MAL-PDT >CT>DCF |
| Study | Characteristics and Intervention | Local Adverse Effects |
|---|---|---|
| Lebwohl et al.1 | See above | Local reactions:-AK, face and scalp: 98%Moderate-intense reactions in a “minority” of casesAK trunk and limbs: 96%70% moderate to intense |
| Study | Characteristics | Intervention | Adherence |
|---|---|---|---|
| Lebwohl et al.1 | See above | See above | >98% |
| Shergill et al.8 | Cross-sectional studyn=305DCF, 5% 5-FU, 5% IMQ, 0.5% 5-FU | Objective: To identify adherence rate and factors conditioning adherence | Percentage of non-adherence or abandonmenta88%Percentage of non-adherence by length of treatment52% in treatments of 3-4 wks duration69% in treatments of 6-8 wks duration71% in treatments of 6-12 wks duration |
Abbreviations: 5-FU, 5-fluorouracil; ALA, 5-aminolaevulinic acid; AK, actinic keratosis; CT, cryotherapy; DCF, diclofenac; IM, ingenol mebutate; IMQ, imiquimod; MAL, methyl aminolaevulinate; PDT, photodynamic therapy; RCT, randomized clinical trial.
It is difficult to perform a direct comparison of the different treatment options for AK due to the heterogeneity of the available studies.4,5Table 2 summarizes the results of studies which, based on the best scientific evidence, analyze the effectiveness of various interventions, including ingenol mebutate. In terms of effectiveness, ingenol mebutate is ranked midway between the most effective treatment (5-fluorouracil) and the least effective options (cryotherapy and diclofenac), achieving results comparable to treatments of intermediate effectiveness (photodynamic therapy and IMQ).6,7 In the case of ingenol mebutate, relative effectiveness is influenced by the site of treatment, with higher rates of clearance for the head region, for which it has proved more effective than 5% IMQ.6
With respect to adherence to treatment, 94% of our patients completed treatment, a percentage similar to that reported by other authors.1 Adherence to treatment with ingenol mebutate is much higher than with other patient-applied topical therapies (Table 2),8 a phenomenon that can be explained by the short treatment cycle required.9
Although localized skin reactions at the site of application of ingenol mebutate are common (>96%), these usually occur after the treatment cycle has been completed and do not, therefore, lead the patients to abandon treatment.1 Local inflammatory reactions in the treated area are also common during the administration of the other topical treatments used to treat AK.10
The retrospective design of the present study has allowed us to describe the characteristics of treatment with ingenol mebutate in clinical practice. However, it also has several limitations. Since no histological analysis is available of the lesions in the treated area, the selection of non-hyperkeratotic or “superficial” AKs was necessarily subjective. This subjectivity may have given rise to minor differences in the severity of the treated area and could therefore have affected our results. In the absence of any exact count of the number of lesions at baseline, there was also a degree of subjectivity in the assessment of whether response was complete or partial. The early assessment of response to treatment (at 2 months) may also have positively influenced our results. Nonetheless, we considered 2 months to be the best moment for this evaluation because it allowed us to assess all the patients in the study at the same time point since the prescribing physicians followed the indications of the Summary of Product Characteristics with respect to the timing of follow-up. Finally, the small number of patients treated makes it difficult to compare our results with other studies.
Our conclusion is that ingenol mebutate is useful in the treatment of AKs and field cancerization, and that the dosage used favors adherence to treatment.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that the procedures followed adhered to the ethical guidelines of the responsible committee on human experimentation and comply with the Declaration of Helsinki of the World Medical Association.
Data confidentialityThe authors declare that they followed the protocols of their institution with respect to the publication of private patient data.
Right to privacy and informed consentThe authors obtained the informed consent of the patients and/or subjects referred to in this article. These documents are in the possession of the corresponding author.
Conflicts of InterestAna Batalla and Ángeles Flórez have received lecture fees from Leo-Pharma Spain for interventions on the topic of actinic keratosis.
The other authors declare that they have no conflicts of interest.
Please cite this article as: Batalla A, Flórez Á, Feal C, Peón G, Abalde MT, Salgado-Boquete L, et al. Respuesta a ingenol mebutato en los pacientes con queratosis actínicas en la práctica clínica. Actas Dermosifiliogr. 2015;106:e55–e61.