Anti-tumor necrosis factor therapy for moderate to severe psoriasis can increase the risk of active tuberculosis in patients who have latent tuberculosis infection (LTBI). The main objective of this study was to estimate the prevalence of LTBI in patients with moderate to severe plaque psoriasis being treated in dermatology clinics in Spain.
Material and methodNon-interventional, cross-sectional, national epidemiological study conducted in Spain in 2011-2012. Patients with moderate to severe plaque psoriasis were included if they had undergone at least one tuberculin skin test (TST) and/or been evaluated with an interferon-γ release assay (IGRA) based on enzyme-linked immunosorbent assay (QuantiFERON TB Gold In-Tube) in the 2 years preceding the study.
ResultsData for 440 patients were valid for analysis. In total, 97.7% of the patients had undergone a TST, with a positive result in 23%. Of the 238 patients in whom the initial result was negative, 5% converted to positive on re-testing for a booster effect. IGRA results were available for 16.8%, 20.5% of them positive. Two of the patients with positive IGRA results had a negative TST. The prevalence of LTBI in the whole sample was 26.6%. The degree of concordance between the TST and the IGRA was moderate (κ=0.516; P<.001).
ConclusionsThe prevalence of LTBI in this study was similar to previous estimates for Spain.
Los agentes biológicos anti-TNF usados para el tratamiento de la psoriasis moderada y grave pueden incrementar el riesgo de desarrollar tuberculosis activa en pacientes con infección tuberculosa latente. El objetivo principal de este estudio fue estimar la prevalencia de infección tuberculosa latente en pacientes con psoriasis en placas moderada y grave en consultas de dermatología en España.
Material y métodoEstudio epidemiológico, no intervencionista, de corte transversal y ámbito nacional, realizado en España en 2011-2012. Se incluyeron pacientes con psoriasis en placas moderada y grave, a los que se les había realizado en los 2 años previos a su inclusión en el estudio al menos una prueba de tuberculina y/o una prueba de liberación de IFN-γ mediante la técnica de ELISA QuantiFERON®-TB gold In Tube.
ResultadosSe incluyeron 440 pacientes evaluables. Se había realizado una prueba de tuberculina al 97,7% de los pacientes, resultando positiva en el 23%. En 238 pacientes con una primera prueba negativa se realizó un booster, que fue positivo en el 5%. Se realizó la determinación del QuantiFERON®-TB al 16,8% de los pacientes, resultando positivo en el 20,5%; en 2 de estos pacientes la prueba de la tuberculina había sido negativa. En el total de la muestra, la prevalencia de infección tuberculosa latente fue del 26,6%. El grado de concordancia entre la prueba de tuberculina y el QuantiFERON®-TB fue medio (índice Kappa=0,516; p<0,001).
ConclusionesLa prevalencia de infección tuberculosa latente estimada en este estudio fue similar a la comunicada previamente en España.
After implementation of the Spanish Society for Rheumatology's 2002 guidelines on managing latent tuberculosis infection (LTBI) in patients treated with antagonists of tumor necrosis factor (anti-TNF agents), the prevalence of active TB fell by 78% in these patients according to a surveillance study of data from the BIOBADASER registry.1 Another analysis of the registry found 34 cases of active TB, all in patients with rheumatoid arthritis treated with infliximab; only 2 of them, however, had started treatment after the 2002 recommendations had been implemented.1 The use of anti-TNF biologics to treat moderate and severe psoriasis in dermatology began in 2004, so the guidelines of the Spanish Academy of Dermatology and Venereology (AEDV)2,3 had already incorporated the earlier recommendations. In spite of the efficacy of the recommended protocols, recent publications show that these patients continue to have higher risk of active TB than control populations or anti-TNF-naive patients; researchers are therefore emphasizing the need to develop more effective strategies for detecting LTBI in Spain.4–6
The incidence of active TB in Spain in 2012, at 13.0 cases per 100000 person-years,7 was higher than rates in surrounding countries.7,8 Recent research also suggests that psoriasis itself may be an independent risk factor for active TB,9,10 and in Spain's psoriasis patients seems to be 7- to 10-fold higher than the general population.5 The main objective of this study was to provide additional information on the prevalence of LTBI in Spanish patients who are candidates for anti-TNF therapy for moderate to severe plaque psoriasis. LTBI screening was based on tuberculin skin tests (TSTs) and/or interferon (IFN)-γ release assays (IGRAs).
Material and MethodsPatientsThis noninterventional, cross-sectional epidemiologic study of cases in 94 dermatology clinics in Spain was carried out in 2011 and 2012. Included were patients at least 18years old with a diagnosis of moderate to severe plaque psoriasis. Within the 2years prior to enrollment, all the patients had undergone TST screening (a first-step test, Mantoux method, and a second-step test if a booster phenomenon was suspected) and/or an IGRA. There were no exclusion criteria. The study was approved by a Spanish national clinical research ethics committee and patients gave their written informed consent to participation.
Variables and Measurement InstrumentsSocial and demographic data recorded were age, gender, weight, and country of birth (in the case of immigrants), place of residence, employment and recreational activities, smoking and alcohol intake, concomitant diseases, personal and family history of TB, and personal history of vaccination with the bacillus Calmette-Guérin (BCG). Clinical data recorded were date of psoriasis diagnosis, clinical form on presentation, family history of psoriasis, markers of disease activity, Psoriasis Area and Severity Index (PASI), the body surface area affected, and the Physician Global Assessment (PGA) score. We also collected data relevant to LTBI screening. An initial Mantoux TST11 result was considered positive12 if an induration ≥5mm was observed, regardless of whether the patient had or had not been vaccinated with BCG. Patients with a first negative TST result were re-tested to rule out a booster phenomenon 7to 10days later, and the results of the second reading were considered definitive. We also recorded the results of IGRAs (QuantiFERON-TB Gold in Tube [QFN-GIT], Cellestis Limited, Carnegie, Victoria, Australia), which detect IFN-γ against Mycobacteriumtuberculosis antigens by enzyme linked immunoassay. Posteroanterior and lateral radiographs were also taken if the researcher considered they would be useful. The study protocol did not specify sputum samples. After active TB was ruled out, a patient was considered to have LTBI on the basis of one or both of the following criteria: a)an induration ≥5mm in diameter on a first- or second-step TST, and/or b)a positive IGRA finding. LTBI prevalence rates were determined for each Spanish autonomous community. Possible LTBI risk factors were explored.
Statistical AnalysisDescriptive statistics of quantitative and qualitative variables were compiled. The t and the Mann-Whitney U tests were used to check for statistically significant differences in quantitative variables. For differences in qualitative variables we used Pearson's χ2 test or the Fisher exact test for 2×2 tables and likelihood ratios for m×n tables. The level of agreement between qualitative variables was expressed by the κ statistic. Factors associated with the presence of LTBI (the dependent variable) were explored by multivariate logistic regression analysis. Estimates were based on a 95% confidence level using SPSS software (version 17.0).
ResultsWe found data for 440 evaluable patients; 67.4%(293/435) were men. The mean(SD) age was 6.6(13.3)years, the mean weight was 80.5(16.3)kg, and 95.9%(418/436) were Caucasian. Psoriasis was moderate in 50.8%(223/439) and severe in 36.4%(160/439) at the time of the baseline visit; the mean time since onset of disease was 18.9(11.0) years. Most patients had plaque psoriasis (90.7%,399/440). Mean clinical scores and data were as follows: PASI,13.3(10.1); affected body surface area, 25.3%(22.0%); and PGA score,3.8(1.6). At the time of screening for LTBI, 59.5%(262/440) were employed, 5.0%(22/440) were immigrants, and 59.9%(263/439) lived in a city or greater metropolitan area. At least 1 concomitant disease was recorded for 43.4%(191/440) of the patients, 32.4%(188/435) were current smokers, and 33.0%(142/430) drank alcohol regularly.
A family history of psoriasis was reported by 56.0%(235/420) of the patients (in parents in 45.1%,106/235), and 1.1%(5/439) had a personal history of TB. Active TB was present in 1.3%(5/396). One of the 5(20%) had pulmonary TB, and 2(40%) had pleural TB at the time of screening. Prior BCG vaccination was reported by 16.5%(57/288) of the patients; a mean of 36.9(10.1)years had passed since vaccination. A trip abroad had been taken in the year prior to LTBI screening by 14.8%(65/440), and 1.8% (8/434) had lived with a relative or worked with someone with active TB (bacilliferous individuals); 3.5%(14/403) had had contact with persons who might have had TB. A chest radiograph was obtained for 88.0% (387/440) of the patients. Signs suggestive of old TB disease were seen in 2.1% (8/386). The most commonly seen signs were calcified hilar lymph nodes (37.5%, 3/8 patients) and pleural thickening with or without calcification (25%, 2/8 patients).
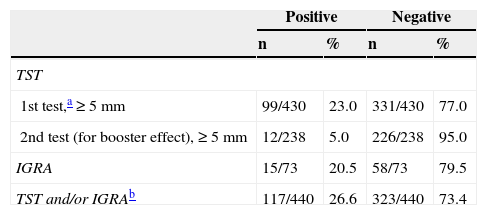
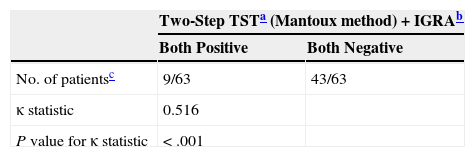
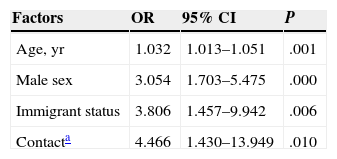
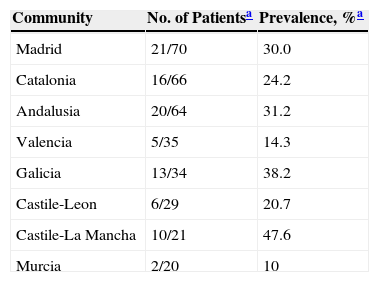
LTBI was screened for by means of only a first-step TST in 97.7% (430/440). In 23% (99/430) of the cases, the result was positive (induration ≥5mm). A second-step TST (for a booster phenomenon) was performed in 71.9% (238/331) of the patients who had had negative first-step TST results, and the second test was positive in 5%(12/238) (Table 1). For 16.8% of the patients (73/440), IGRA results were also available (positive for 20.5%, 15/73). Two of these patients with positive IGRA results had had a negative TST, and 4 of them had not undergone TST screening (Table 1). The level of agreement between the TST and IGRA results was moderate (κ,0.516, P<.001) (Table 2). The prevalence of LTBI was significantly higher in men (31.7%,93/293) than in women (15.5%,22/142) (P<.001). It was also higher in BCG-vaccinated patients (35.1%,20/57) than in unvaccinated ones (21.5%,62/288) (P<.05). These percentages for vaccinated and unvaccinated patients, respectively, by screening test, were as follows: first TST, 29.6% and 16.6% (P=.100); second TST to check for a booster effect, 8.8% and 2.5% (P=.076); and IGRA, 25% and 14.9% (P=.358). The levels of agreement between the TST and IGRA results were not significantly different between the total population and the subpopulation of BCG-vaccinated patients (κ,0.409; P<.067) or the subpopulation of unvaccinated patients (κ,0.599; P<.0001). The overall prevalence of LTBI in our series, considering those with either a positive TST or IGRA screening result was 26.6% (117/440). Factors that were statistically significantly related to a finding of LTBI in the multivariate regression analysis are summarized in Table 3. The prevalence by Spanish autonomous community is shown in Table 4 for those communities with information for more than 20 patients in the study.
Estimated Prevalence of Latent Tuberculosis Infection, According to Screening Test.
| Positive | Negative | |||
|---|---|---|---|---|
| n | % | n | % | |
| TST | ||||
| 1st test,a ≥5mm | 99/430 | 23.0 | 331/430 | 77.0 |
| 2nd test (for booster effect), ≥5mm | 12/238 | 5.0 | 226/238 | 95.0 |
| IGRA | 15/73 | 20.5 | 58/73 | 79.5 |
| TST and/or IGRAb | 117/440 | 26.6 | 323/440 | 73.4 |
Abbreviations: IGRA, interferon-γ release assay; TST, tuberculin skin test (Mantoux method).
Screening for Latent Tuberculosis Infection: Level of Agreement Between TST and IGRA Screening.
| Two-Step TSTa (Mantoux method)+IGRAb | ||
|---|---|---|
| Both Positive | Both Negative | |
| No. of patientsc | 9/63 | 43/63 |
| κ statistic | 0.516 | |
| P value for κ statistic | <.001 | |
Abbreviations: IGRA, interferon-γ release assay; TST, tuberculin skin test.
Factors Positively Associated With Presence of Latent Tuberculosis Infection in Multivariate Regression Analysis.
| Factors | OR | 95% CI | P |
|---|---|---|---|
| Age, yr | 1.032 | 1.013–1.051 | .001 |
| Male sex | 3.054 | 1.703–5.475 | .000 |
| Immigrant status | 3.806 | 1.457–9.942 | .006 |
| Contacta | 4.466 | 1.430–13.949 | .010 |
Abbreviation: OR, odds ratio.
Prevalence of Latent Tuberculosis Infection, by Spanish Autonomous Community.
| Community | No. of Patientsa | Prevalence, %a |
|---|---|---|
| Madrid | 21/70 | 30.0 |
| Catalonia | 16/66 | 24.2 |
| Andalusia | 20/64 | 31.2 |
| Valencia | 5/35 | 14.3 |
| Galicia | 13/34 | 38.2 |
| Castile-Leon | 6/29 | 20.7 |
| Castile-La Mancha | 10/21 | 47.6 |
| Murcia | 2/20 | 10 |
Data in the second column are number of patients with latent tuberculosis infections/the total number of patients with information from the autonomous community named. Prevalence rates were calculated for communities with ≥20 patients included in the analysis. By way of comparison, the rates reported for these communities in 2012 were as follows7: Madrid, 12.2%; Catalonia, 16.8%; Andalusia, 10.7%; Valencia, 10.1%; Galicia, 24.6%; Castile-Leon, 14.3%; Castile-La Mancha, 8.4%; and Murcia, 10.1%.
The prevalence of LTBI we have estimated for Spain (26.6%) is consistent with rates previously published.5,13 The estimate based on the BIOBADADERM registry was 20%, and 17% of the patients with the diagnosis had not been screened according to recommendations; failure to order a second TST to rule out a booster effect was the most common type of noncompliance.5 The prevalence of a diagnosis of LTBI increased by 5% in our study after a second-step TST. The higher prevalence (29%) reported by Sánchez-Moya and Dauden13 can be attributed to the stricter compliance with guidelines in their study, and their rate probably more accurately reflects the reality in our dermatology practices. Ours is the first multicenter study in Spain to estimate the prevalence of LTBI based on combined results of 2-step TST and IGRA screening. A recent single-center Spanish study screened for LTBI in 103 patients with moderate to severe psoriasis who were on a systemic immunosuppressant or about to initiate treatment with one. Screening was accomplished with 2 IGRAS (the QFN-GIT and the T-SPOT.TB kit) and the TST. Prevalence rates were 16.5%, 17.5%, and 8.7% with the 3 techniques, respectively, and rose to 24.3% when the results for all of them were combined.14 Those rates were consistent with the previously mentioned reports and with our findings. The rates we report are higher than those seen in other European countries in which IGRAs were used.15,16 However, given the heterogeneity of the published literature (regarding screening techniques and geographic variability), it is difficult to compare prevalence rates.
A major problem of TST screening is low specificity, given that results are positive in individuals who have been vaccinated with BCG as well as those who have become sensitized from exposure to nontuberculous mycobacteria. In addition, the TST has low sensitivity in patients with altered cellular immunity.17 Yet another problem is high interindividual variability in the interpretation of results. In contrast, IGRAs are unaffected by BCG and most environmental mycobacteria, and their interpretation is invariable.18 Unlike the TST, IGRAs seem to have higher sensitivity, particularly in immunocompromised individuals or populations with high rates of BCG-vaccinated individuals.19 The QFN-GIT IGRA was used in 16.8% of the patients in our study and 20.5% of them had positive results for LTBI. Two of the patients with positive IGRA results had negative TST findings. We saw moderate agreement between the 2 screening tools (κ,0.516; P<.001). That level of agreement was slightly lower than the one reported by Prignano et al.20 in a series of 267 dermatology patients (κ,0.69; P<.0001) but higher than the level reported by Gisondi et al.21 (κ,0.15). We concur with most of the literature we reviewed in thinking that IGRAs might be the first-choice technique for LTBI screening in these patients. However, given the higher prevalence of LTBI in our series, in which patients had a history of BCG vaccination, as well as the moderate agreement we observed in both the overall series and the subgroups with and without a vaccination history, it seems advisable to recommend the combination of TST and IGRA screening in our psoriasis patients who are candidates for anti-TNF therapy, as suggested elsewhere.21,22 Our multivariate regression analysis revealed that LTBI was associated with being an immigrant or having had contact with persons with possibly active TB in the year before screening, and we emphasize the need to record this information when taking a patient's medical history. We have only reported LTBI data for 8 of Spain's autonomous communities, specifically those for which information for at least 20 patients was available. Even so, given the small samples for the communities we do report, we are unable to compare our results with the 2012 data reported in 2013 by the Spanish center for epidemiological statistics.7
Relevant limitations to bear in mind when interpreting this study include especially the lack of information from patient follow-up after anti-TNF therapy was started. Since information on the prophylactic treatment prescribed is unknown, we cannot evaluate the incidence of active TB in the years following start of therapy. The inclusion of data from a large number of centers is also a limitation that affects the evaluation of TST results, given high variability in how centers interpreted those results. We have no information on when the results were read in each center or the type of commercial preparation used. The centers’ methods for implementing IGRA analyses are also unknown. We have no information about the sequence of TST and IGRA screening in patients who had results from both tests. Information about the immunocompetence of patients or their possible use of immunosuppressants at the time of screening is also missing. A better understanding of these variables would undoubtedly contribute to explaining at least partially the moderate level of agreement between the 2 screening tests.
With these limitations in mind, we conclude that the prevalence of LTBI can be estimated to be about 26.6% based on the combined results of TST and IGRA (QFN-GIT) screening of a large series of 440 patients with moderate to severe psoriasis who were candidates for biologic therapy. This prevalence is consistent with previous reports for the situation in Spain.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Data confidentialityThe authors declare that they followed their hospitals’ regulations regarding the publication of patient information and that written informed consent for voluntary participation was obtained for all patients.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in this article. The signed forms are in the possession of the corresponding author.
FundingThis study was sponsored by Pfizer España.
Conflicts of InterestM. Ribera declares that he has received research grants and payments, consultancy fees, and training fees from the following companies: Abbott, Janssen, LEO Pharma, MSD, Novartis, and Pfizer. Ander Zulaica declares that he has received fees from the following companies for participating in clinical trials, giving training conferences, or consulting: Abbvie, Pfizer, Janssen, MSD, and Novartis. Conrad Pujol declares that he has received fees from the following companies for participating in clinical trials, giving training conferences, or consulting: Abbvie, Pfizer, Janssen, MSD, and Novartis. Maria Luisa Alonso declares that she has received fees for serving as an expert consultant, for participating in clinical trials, or giving conferences for Pfizer. Isabel Maria Rodríguez declares that she has received fees from the following companies for participating in clinical trials, giving training conferences, or consulting: Abbvie, Pfizer, Janssen, and MSD. Carmen García Calvo works as a consultant to the medical department of Pfizer España.
Maria Luz Samaniego, of Trial Form Support, Madrid, Spain, provided support in carrying out the statistical analyses.
Please cite this article as: Ribera M, Zulaica A, Pujol C, Alonso ML, Rodriguez IM, Garcia-Calvo C, et al. Estimación de la prevalencia de infección tuberculosa latente en pacientes con psoriasis en placas moderada a grave en España. Estudio Latent. Actas Dermosifiliogr. 2015;106:823–829.