Bullous pemphigoid (BP) is an autoimmune subepidermal bullous disease in which autoantibodies are directed against components of the basement membrane. Most of these antibodies belong to the immunoglobulin G class and bind principally to 2 hemidesmosomal proteins: the 180-kD antigen (BP180) and the 230-kD antigen (BP230). It is the most common blistering disease in the adult population in developed countries, with an estimated incidence in Spain of 0.2 to 3 cases per 100,000 inhabitants per year. The disease primarily affects older people, although it can also occur in young people and even in children. In recent years, advances in clinical practice have led to a better understanding and improved management of this disorder. These advances include new diagnostic techniques, such as enzyme-linked immunosorbent assay for BP180 and new drugs for the treatment of BP, with diverse therapeutic targets. There is, however, still no international consensus on guidelines for the management of BP. This article is an updated review of the scientific literature on the treatment of BP. It focuses primarily on evidence-based recommendations and is written from a practical standpoint based on experience in the routine management of this disease.
El penfigoide ampolloso (PA) es una enfermedad ampollosa autoinmune causada por anticuerpos dirigidos contra componentes de la membrana basal. La mayoría de estos anticuerpos son de clase IgG y se unen principalmente a 2 proteínas hemidesmosómicas, los antígenos BP180 y BP230.
Se trata de la enfermedad ampollosa más frecuente en los países desarrollados en la población adulta, con una incidencia estimada en nuestro medio de 0,2 a 3 casos nuevos por cada 100.000 habitantes. Afecta principalmente a pacientes ancianos, aunque puede presentarse en jóvenes e incluso niños.
En los últimos años hemos incorporado a la práctica clínica nuevas técnicas diagnósticas (ELISA para BP180) y nuevos fármacos con distintas dianas terapéuticas en el tratamiento del PA. Esto ha permitido un avance en el conocimiento y el manejo de esta entidad.
A pesar de ello, no existen a día de hoy unas guías consensuadas internacionalmente sobre el manejo del PA.
Este artículo es una revisión actualizada de la literatura científica sobre el tratamiento de esta enfermedad, destacando las recomendaciones basadas en la evidencia y desde un punto de vista práctico basado en la experiencia del manejo diario de estos pacientes.
Bullous pemphigoid (BP) is an autoimmune blistering disorder characterized by the formation of autoantibodies directed against components of the basement membrane. Most of these antibodies belong to the immunoglobulin (Ig) G class and bind primarily to 2 hemidesmosomal proteins: the 180-kD antigen (BP180) and the 230-kD antigen (BP230). There is experimental and clinical evidence that these autoantibodies—and particularly those targeting BP180—are responsible for causing the blisters and therefore the disease.
Several recent articles have published interesting recommendations on the management of BP,1–4 but there are no international consensus guidelines, and furthermore, there are no updated guidelines available in Spanish.
In this paper, we review the current scientific literature on the treatment of BP and highlight recommended approaches based on available evidence and experiences from routine clinical practice.
EpidemiologyBP is the most common blistering disorder in the adult population in developed countries, with an estimated incidence of 0.2 to 3 cases per 100 000 inhabitants a year. While recently published studies have pointed to an increase in recent years,5 a French study published in just 2012 reported an incidence of 21.7 cases per million inhabitants a year.6 The disease affects a similar proportion of men and women. Most patients are over 75 years old, but young adults and even children can also develop BP. Several cases reporting an association between BP and different malignant diseases have been published, but in one case-control study with matching for age and sex no increased risk for malignancy was observed in BP patients.7
PathogenesisThe pathogenesis of BP is essentially determined by 2 components:
- 1.
An immunologic component, determined by the presence of antibodies directed against basal keratinocytic hemidesmosomal proteins (mainly antigens BP180 and BP230).
- 2.
An inflammatory component, which plays a greater role in BP than in other autoimmune blistering disorders. It is driven by the action of polymorphonuclear cells (neutrophils, eosinophils) activated by the Fc portion of the antibodies, leading to the release of proteolytic enzymes and consequent damage of the dermal-epidermal junction.
Until recently, anti-BP180 IgG antibodies were believed to be the only pathogenic antibodies in BP, but specific IgE-type anti-BP180 antibodies have also been identified. These antibodies have recently been attributed a pathogenic role in the formation of BP skin lesions in animal models,8–10 and similarly to anti-BP180 IgG antibodies, it is thought their levels might be correlated with disease activity.11
Numerous drugs (spironolactone, furosemide, bumetanide, D-penicillamine, amoxicillin, ciprofloxacin, potassium iodide, gold salts, and captopril) have been proposed as possible triggers of BP, but the exact mechanisms involved are unknown. One theory is that these drugs might alter the immune response or the antigens of the basement membrane in patients with a certain genetic predisposition.3 Furthermore, there have been reports of BP induced by phototherapy, radiation therapy, vaccines, and viral infections, as well as cases in transplant recipients with acute or chronic rejection. Detection of these possible triggers depends largely on a thorough clinical history, particularly in elderly patients, who are frequently exposed to multiple drugs. The control or elimination of the trigger, where possible, can greatly help in the management of certain patients.
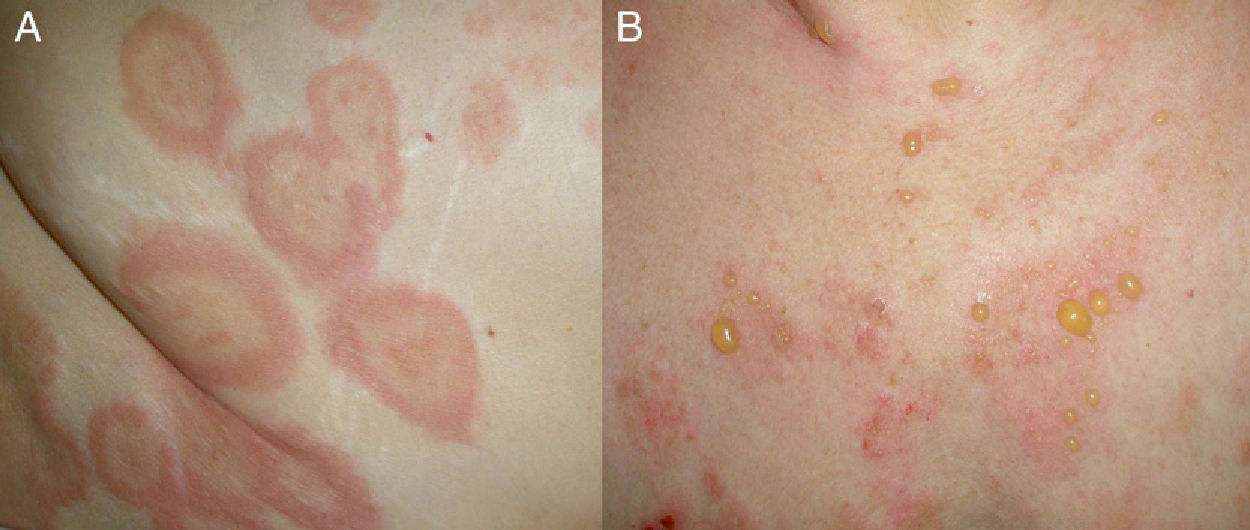
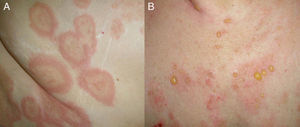
Clinical PresentationClinically, BP is characterized by the appearance of highly pruritic urticaria-like or eczematous lesions on which tense blisters may develop after a variable period of time (Fig. 1). Some patients only develop intense itching, and the only lesions visible in these cases are excoriations. Pemphigoid should therefore be considered in the differential diagnosis of chronic itching in an elderly patient. Patients initially develop vesicles, but these may progress to large blisters that are typically filled with a clear fluid or blood. They are located predominantly on the trunk and the flexor surface of the extremities, and rarely affect the head or neck. The Nikolsky sign is negative, although a positive result does not rule out a diagnosis of BP. In our practice, we have seen some cases of BP with a positive Nikolsky sign.
The blisters heal without scarring, although postinflammatory hyperpigmentation is common. Atypical, localized forms of BP also exist, including dyshidrosiform pemphigoid (affecting the palms and soles), erythrodermic pemphigoid, nodular pemphigoid (similar to nodular prurigo), lichen planus pemphigoides, pemphigoid vegetans, and variants confined to irradiated sites. Mucosal involvement is rare, and when it does occur, it is generally mild and tends to be limited to the mouth.
DiagnosisBP is diagnosed on the basis of clinical, histologic, and immunologic findings. There are no fixed diagnostic criteria, unlike in other bullous disorders, although one group of French authors proposed that the following clinical predictors of BP should be evaluated in a patient with a bullous eruption12: absence of atrophic scars, absence of head and neck involvement, absence of mucosal involvement, and age greater than 70 years. Based on the authors’ calculations, the presence of 3 of these 4 diagnostic criteria for BP had a sensitivity of 90%, a specificity of 83%, and a positive predictive value of 95%.
Two skin biopsies should be taken when BP is suspected.
- 1.
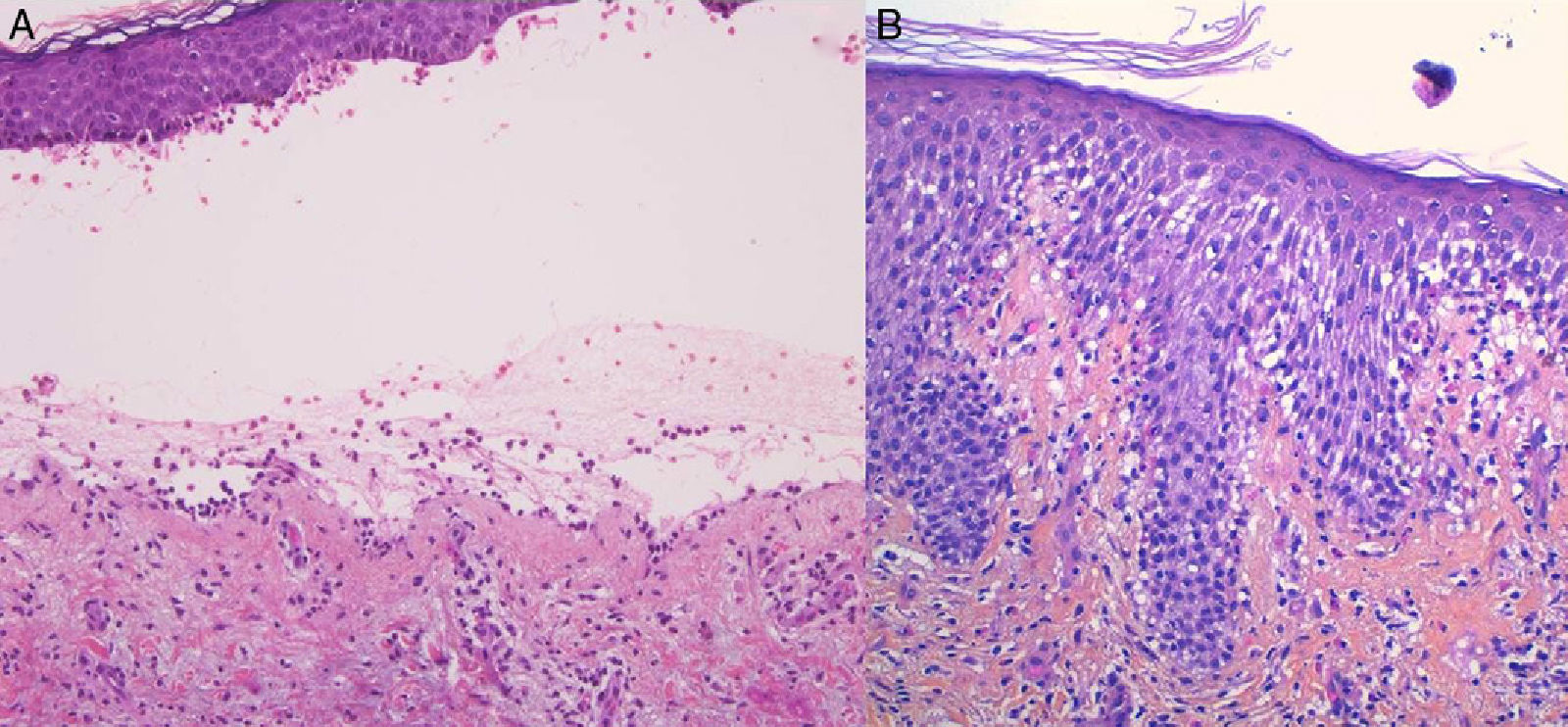
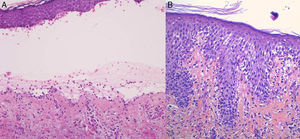
For the standard histologic study, a sample should be taken from a recent vesicular lesion (to avoid reepithelialization phenomena) and fixed in formaldehyde. Typical findings include a subepidermal blister with a mixed superficial perivascular inflammatory infiltrate with abundant eosinophils (Fig. 2A). Vasculitis is not seen and inflammatory infiltrates do not generally affect the deep reticular dermis or the subcutaneous tissue, unlike infiltrates seen in arthropod bite reactions. Eosinophilic spongiosis is a characteristic feature of early or prebullous lesions (Fig. 2B). While not pathognomic, the presence of eosinophilic spongiosis in a patient over 70 years in association with eosinophils along the basement membrane is highly suggestive of BP.
Figure 2.A, Subepidermal blister with a mixed superficial perivascular inflammatory infiltrate with abundant eosinophils (hematoxylin-eosin, original magnification ×210). B, Eosinophilic spongiosis, a frequent histologic feature in early bullous pemphigoid lesions (hematoxylin-eosin, original magnification ×210).
- 2.
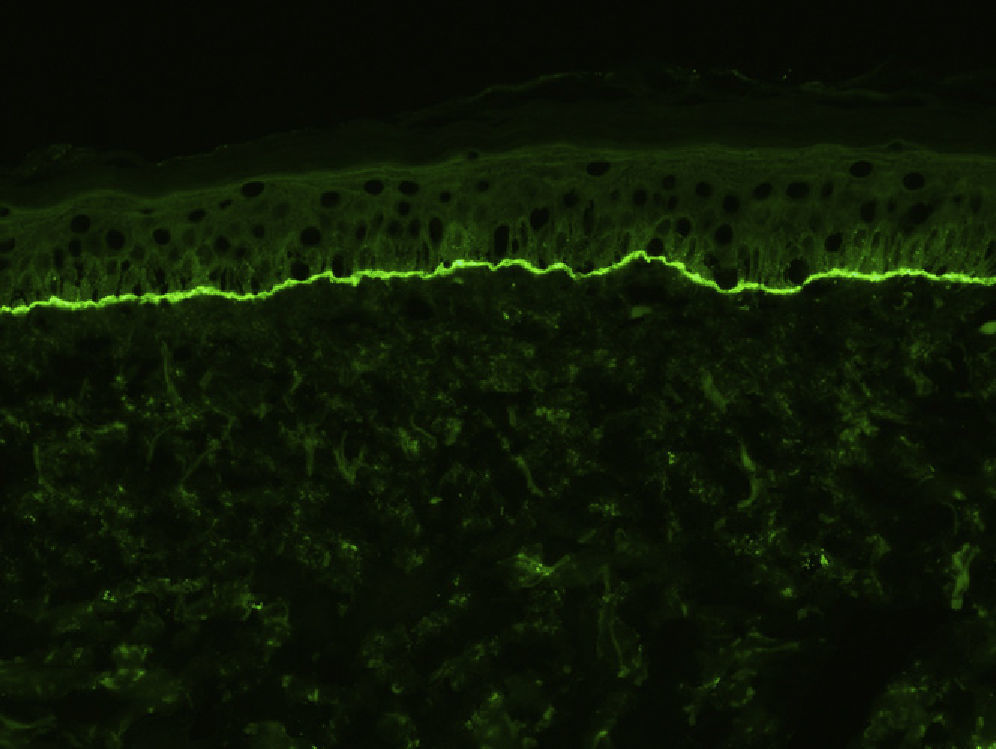
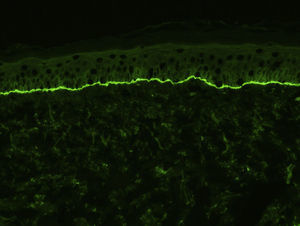
For the direct immunofluorescence (DIF) study, a second biopsy sample should be taken from healthy perilesional skin or from inflamed skin without vesicles or blisters (to avoid the false negatives that can occur in such cases). Biopsies should be fresh-frozen immediately or placed in saline solution or Michel's transport medium. The best results have been found with biopsies stored in saline for 12 to 24 hours.13 Samples that may take longer than 24 hours to reach the laboratory should be placed in Michel's transport medium, which permits storage for several weeks, even at room temperature.14 Characteristic DIF findings are linear deposits of IgG and C3 along the basement membrane, although there may also be less intense deposits of other immunoglobulins, such as IgA and IgM (Fig. 3). C3 deposits are generally more intense than IgG deposits. A predominance of IgG deposit intensity would suggest another diagnosis, such as epidermolysis bullosa acquisita. A small percentage of patients with BP have C3 deposits only. Another histologic feature described as characteristic of BP is an n-serrated pattern of IgG deposition (contrasting with the u-serrated pattern seen in epidermolysis bullosa acquisita),15 although in our experience, this pattern is not often observed.
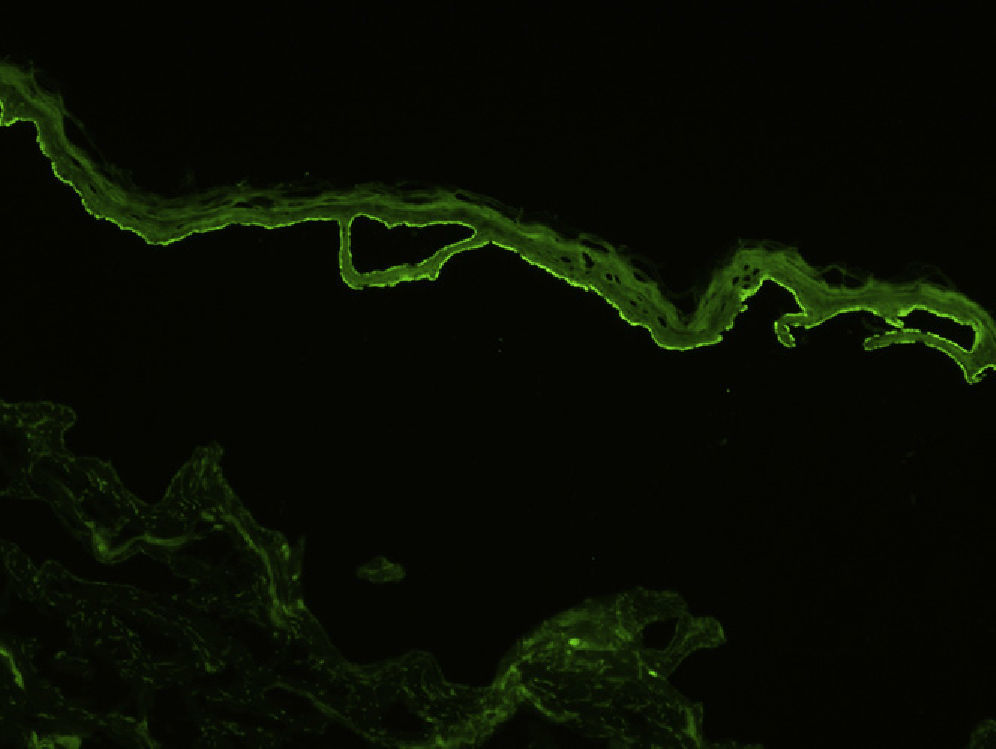
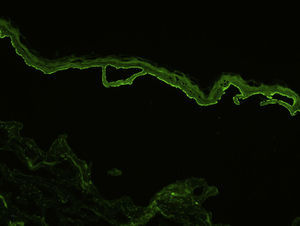
Indirect immunofluorescence (IDIF) is used to test for circulating anti-basement membrane zone antibodies in peripheral blood or blister fluid. The test can be performed with monkey esophagus substrate, or better still with human skin treated with 1 M sodium chloride solution (the salt-split technique), which separates the basement membrane from the lamina lucida (Fig. 4). The salt-split technique is more sensitive and also permits BP antibodies (deposited primarily at the epidermal side of the induced blister) to be distinguished from antibodies encountered in other diseases such as epidermolysis bullosa acquisita. In some patients with a negative IDIF result, DIF may be performed on biopsied skin processed using the salt-split technique.16
Finally, several tests exist to determine the antigenic specificity of autoantibodies. Some of these, such as the immunoblot assay, reveal the presence of antibodies against proteins with a molecular weight of 180 and/or 230 kD corresponding to the antigens BP180 and BP230 (when epidermal extracts are used) or of antibodies against the NC16A portion of the BP180 antigen (when recombinant proteins are used). These techniques, however, are reserved for investigational purposes in highly specialized laboratories. Currently, the most widely used technique is the enzyme-linked immunosorbent assay (ELISA), which detects circulating antibodies against the 2 BP antigens—BP180 and BP230—in a large proportion of patients. Quantitative detection of antibodies is useful for monitoring patients, as anti-BP180 antibody levels have been found to correlate with disease activity. This assay is sold by 2 companies: Euroimmun in Lübeck, Germany and MBL in Nagoya, Japan. Finally, in recent years, Euroimmun has been working with the dermatology department of the University of Lübeck to create tissue biochips consisting of a panel of substrates, including monkey esophagus, human skin separated with 1 M sodium chloride solution, and cells transfected with different antigens and protein aggregates that serve as a substrate for IDIF. These biochips enable the direct identification of target antigens in BP and other blistering disorders.
Differential DiagnosisBlistering diseases in the pemphigoid group are generally clinically identifiable by the presence of fragile blisters that break easily and have a positive Nikolsky sign. Diagnosis is confirmed by histology (intraepidermal blister in pemphigus and subepidermal blister in BP) and immune studies.
It is important to use clinical, histologic, and DIF and IDIF findings to differentiate BP from other diseases with subepidermal blisters, such as dermatitis herpetiforme, epidermolysis bullosa acquisita, linear IgA dermatosis, or mucous membrane pemphigoid. Other even less common diseases, such as anti-laminin-332 mucous membrane pemphigoid and anti-laminin gamma-1 pemphigoid, should also be considered. In some patients, it may be necessary to determine the antigenic specificity of the autoantibodies.
Other conditions such as urticaria, eczema, urticarial vasculitis, erythema multiforme, toxic drug reactions, scabies, and certain viral rashes, (especially in adults) may have a similar clinical presentation to that of early BP lesions. Histology, immune studies, blood tests, and cultures can aid in the differential diagnosis.
PrognosisBullous pemphigoid is normally self-limiting, but it can last for several years (generally less than 5). Prior to the introduction of corticosteroids, BP had an associated mortality of 24%,17 but rates reported since have varied widely (from 6% to 40%). A recent French study of 502 patients, the largest to date, reported a 1-year mortality of 38%, which is 6 times higher than the rate for the general population.6 These data are inconsistent with our experience. In a retrospective review of 101 patients from our hospital, we observed a mortality of 13% during the first year, and found that advanced age (> 80 years) was the only predictor of death.18
Pruritus can severely impair patient quality of life. Furthermore, erosive lesions serve as a potential entry point for infection, and extensive lesions predispose patients to a considerable loss of liquids and electrolytes, as well as impaired thermoregulation. It is important to remember that BP typically affects older patients, who often have concomitant diseases and are taking several drugs. They are therefore more vulnerable and prone to complications and adverse drug reactions.
Additional TestsVarious tests should be requested in patients with a suspected or confirmed diagnosis of BP, preferably before initiation of treatment.
- 1.
Laboratory tests including complete blood count, biochemistry profile, and liver and kidney function
- 2.
Serology for hepatitis virus C (HVC), HVB, human immunodeficiency virus, and varicella-zoster virus
- 3.
Thiopurine S-methyltransferase (TPMT) activity if azathioprine is to be administered
- 4.
Glucose-6-phosphate dehydrogenase (G6PDH) activity if sulfone is to be administered
- 5.
Blood pressure
- 6.
Chest radiograph
- 7.
Mantoux test (purified protein derivative)
Three types of drugs, with distinct mechanisms of action, are used to treat BP:
- 1.
Anti-inflammatory drugs such as topical corticosteroids, sulfone, sulfamides, or antibiotics with anti-inflammatory properties such as tetracyclines
- 2.
Drugs designed to reduce the production of pathogenic antibodies, such as systemic corticosteroids, azathioprine, mycophenolate, cyclophosphamide, methotrexate, ciclosporin, and rituximab
- 3.
Treatments that increase the elimination of pathogenic antibodies from the serum of patients, such as high-dose intravenous immunoglobulin (IVIG) therapy and therapeutic plasma exchange
Although there are no consensus guidelines on the management of bullous pemphigoid,19 when deciding on a treatment approach, it is important to remember that BP is associated with high morbidity (although not as high as in other blistering disorders such as pemphigus vulgaris) and affects mainly elderly patients.
It is generally preferable to choose drugs with the lowest toxicity possible and to target the inflammatory component of the disease.20 As in other autoimmune diseases, treatment does not cure BP but rather suppresses disease activity. In the case of localized lesions, high-potency topical corticosteroids may be sufficient to control the disease and would therefore be treatment of choice.21,22
Practical ConsiderationsIn practice, the choice of treatment and general management of the disease will largely depend on the patient. Several considerations need to be taken into account when dealing with special populations, such as elderly patients or children.
Elderly PatientsElderly patients tend to have age-related immune system impairment and comorbidities and therefore the recommendation to avoid immunosuppressive drugs is even more important in this group of patients.
It is also important to evaluate and take into account each patient's capacity and level of dependency in terms of activities of daily living, as well as their environment (e.g., do they live with their family or in a care home) and the support or help available. These aspects may determine the choice of treatment. For instance, topical treatments are difficult to apply for a person with reduced mobility or who does not have help. Scales such as the Karnofsky Performance Scale Index (originally developed for patients with cancer) can be used to evaluate the patient's general health and guide the assessment.
Furthermore, comorbidities and drug use (which are both common in elderly patients) should be carefully noted down in the initial clinical history, as should any changes that occur during the course of the disease, to avoid prescribing contraindicated drugs or causing unwanted drug interactions.
It should also be recalled that elderly patients may have impaired or altered cognitive functions. In such cases, it can be difficult to evaluate certain clinical aspects, such as pruritus or drug tolerance, and to give instructions about treatment. In our experience, it is important to adequately inform, support, and engage relatives or caretakers in the control of the disease.
ChildrenChildhood BP typically has a better prognosis and a less aggressive course than adult BP.23 The use of toxic drugs and systemic corticosteroids should be minimized due to their adverse effects, particularly in terms of their potential interaction with growth and development. If systemic corticosteroids are to be used, data on height and percentiles should be recorded at the outset to control for changes during follow-up. In our practice, we have seen cases of aggressive, difficult-to-manage BP in children in whom immunosuppressants and systemic corticosteroids were needed to achieve control. In our opinion, management by a multidisciplinary team, including pediatricians, has many benefits. Systemic corticosteroids should not be withdrawn in a child without first performing an ACTH test to check that the adrenal glands are functioning correctly and protect against the risk of acute adrenal insufficiency.
It should also be noted that topical corticosteroids can cause complications due to systemic absorption (which is increased in children), particularly in the case of extensive or mucosal lesions.
Topical TreatmentGood skin hygiene with thorough daily cleansing of skin and mucous membranes is important in all patients with BP. We also recommend keeping an approximate count of lesions. The lesions should be cleansed before the application of the topical treatment. Blisters can be drained by piercing the base of the lesion with a sterile needle or blade. Application of an antiseptic solution, such as eosin 2% or chlorhexidine 0.5%, on erosive lesions reduces the risk of secondary infection. If such an infection does occur, topical antibiotics such as fusidic acid and mupirocin can be used. A course of systemic antibiotics must be started when deep cutaneous infection (cellulitis) is suspected.
Monitoring and Therapeutic ManagementUntil recently, treatment decisions in BP were guided by clinical assessment. Unless contraindicated, systemic corticosteroids are generally the treatment of choice in patients in whom topical treatment is insufficient or difficult to apply. In most cases, they produce clinical improvement within a few weeks, enabling a gradual reduction in dosage and in many cases withdrawal after 6 to 10 months.20 Adjuvant medication is indicated in cases of refractory BP or in patients who are dependent on high-dose systemic corticosteroids. The aim of adjuvant therapy is to achieve an acceptable clinical response and reduce the systemic corticosteroid dose. There is no, however, no consensus on how or when treatment should be modified in BP.
In our experience, apart from clinical evaluation, selective quantification of circulating anti-BP180 antibodies by ELISA is a useful tool for monitoring patients, as antibody levels are correlated with disease activity and can facilitate more accurate treatment decisions.
Presuming that the technique is available, we recommend measuring anti-BP180 antibody levels at the time of diagnosis to use as a reference for monitoring progress.
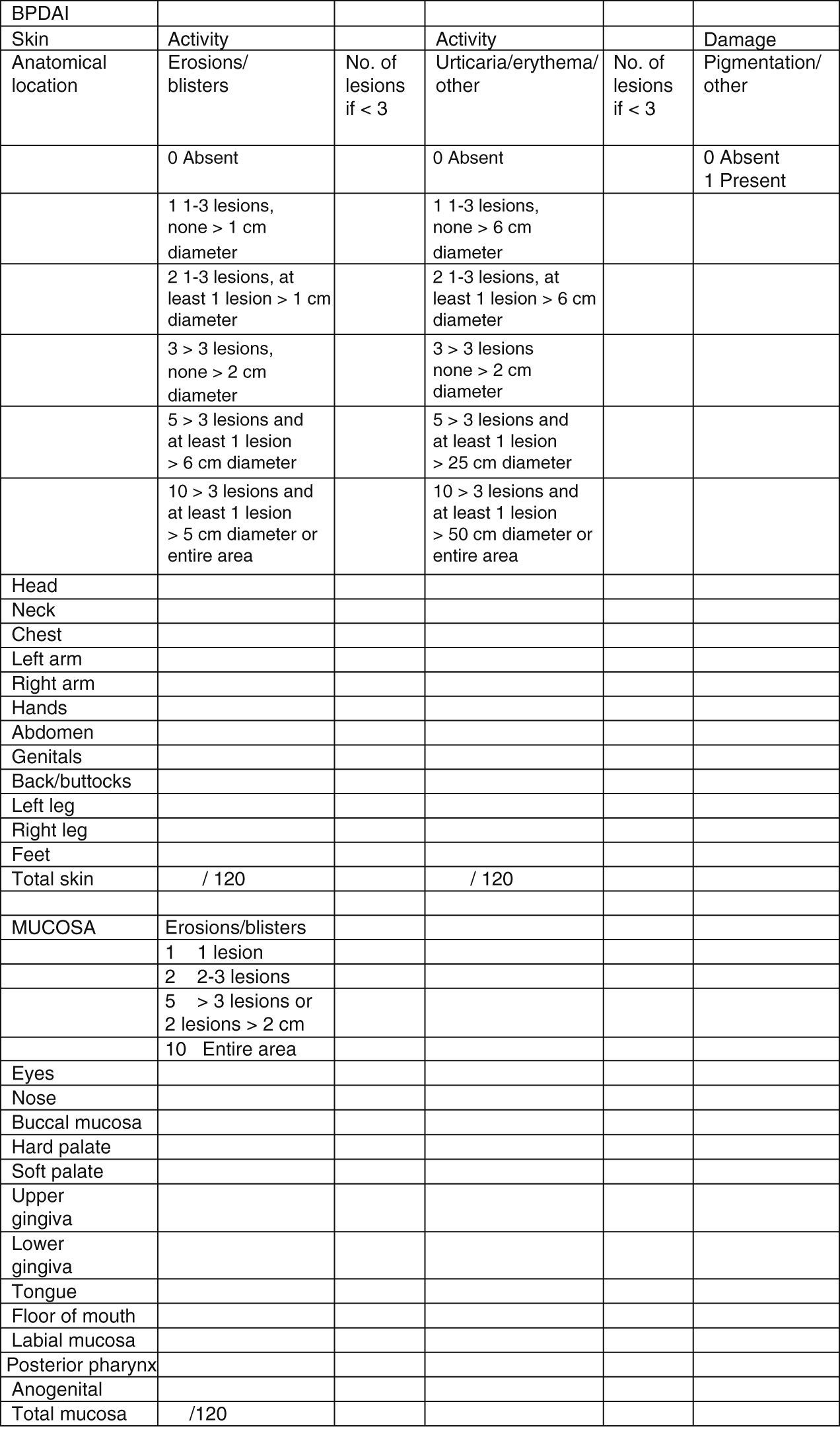
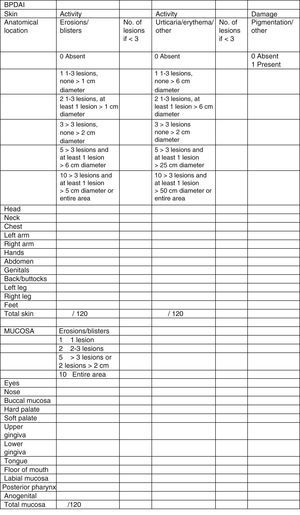
Clinical status can also be assessed using a range of objective measurement tools. The Bullous Pemphigoid Disease Area Index (BPDAI) (Fig. 5), like the Pemphigus Disease Area Index (PDAI), provides a separate measurement of skin and mucosal involvement and takes into account both lesion type and location.24
Bullous Pemphigoid Disease Area Index (BPDAI): Objective measure of cutaneous and mucosal involvement in bullous pemphigoid. Source: Murrell et al.24
The Autoimmune Bullous Skin Disorder Intensity Score (ABSIS) (Fig. 6), originally designed for pemphigus vulgaris, also distinguishes between cutaneous and mucosal lesions and uses a scoring system based on the type and extent of lesions.25 These tools help to minimize the subjectivity of clinical evaluation, and are very useful for reliably comparing results within the same patient, within a group of patients, or, importantly, between patients from different studies.
Autoimmune Bullous Skin Disorder Intensity Score (ABSIS): Objective measure of clinical activity that can be used for bullous pemphigoid. BSA indicates body surface area. Adapted from: Pfütze et al.25
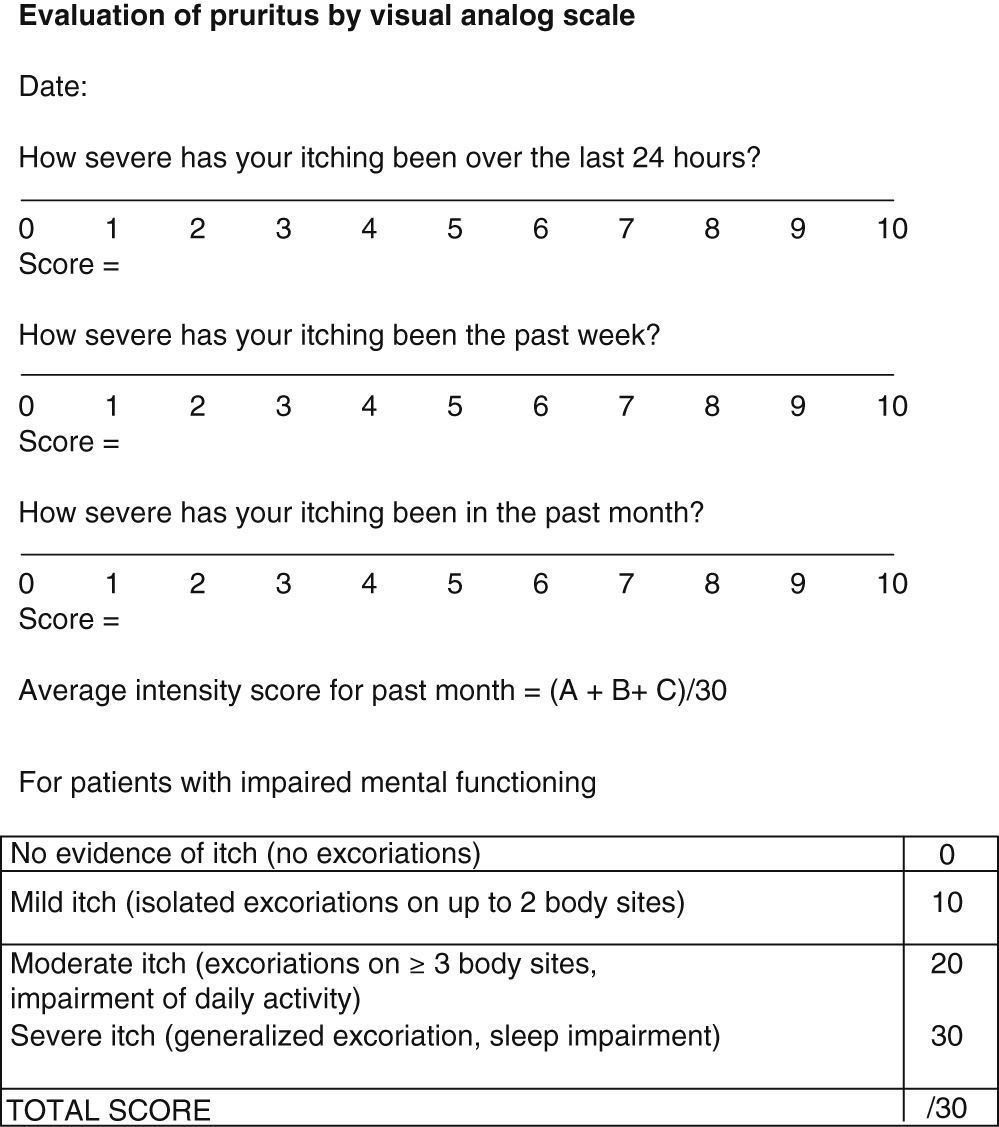
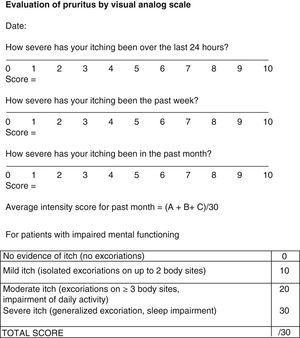
Pruritus is even more subjective and difficult to evaluate. However, it has an impact on patient quality of life and therefore needs to be assessed. The BPDAI pruritus visual analog scale (VAS) (Fig. 7) rates itching severity over the past 24 hours, the past week, and the past month on a scale of 0 to 10.24 For patients unable to use the VAS, severity can be inferred from observation of excoriations from scratching (Fig. 7).24
Visual analog scale for evaluation of pruritus. Source: Murrell et al.24
In response to the need to compare outcomes from different studies of BP, a panel of experts recently produced a set of useful criteria to aid in the monitoring of disease activity.24 These criteria are shown in Table 1.24
Accepted Evaluation Criteria for Bullous Pemphigoid (BP).
| Early observation points | |
| Baseline | Day that BP therapy is started by physician. |
| Control of disease activity | Time at which established lesions begin to heal or pruritic symptoms start to abate and new lesions cease to form. |
| Time to control of disease activity | Time interval from baseline to control of disease activity. |
| End of consolidation phase | Time at which no new lesions have developed for minimum of 2 wk and approximately 80% of lesions have healed and pruritic symptoms are minimal. |
| Transient lesions | New lesions that heal within 1 wk or pruritus lasting <1 wk and clearing without treatment. |
| Nontransient lesions | New lesions that do not heal within 1 wk or pruritus continuing >1 wk with or without treatment. |
| Complete remission during tapering | Absence of nontransient lesions while patient is receiving more than minimal therapy. |
| Late observation points | |
| Minimal therapy | ≤0.1 mg/kg/d of prednisone (or equivalent) or 20g/wk of clobetasol propionate and/or minimal adjuvant or maintenance therapy. |
| Minimal adjuvant therapy and/or maintenance therapy | Following doses or less: methotrexate 5mg/wk; azathioprine 0.7mg/kg/d (with normal thiopurine s-methyltransferase levels); mycophenolate mofetil 500mg/d; mycophenolic acid 360mg/d; or dapsone 50mg/d. |
| Partial remission on minimal therapy | Presence of transient new lesions that heal within 1 wk while patient is receiving minimal therapy for at least 2 mo. |
| Complete remission on minimal therapy | Absence of new transient or established lesions or pruritus while patient is receiving minimal therapy for at least 2 mo. |
| Partial remission off therapy | Presence of transient new lesions that heal within 1 wk without treatment while patient is off all BP therapy for at least 2 mo. |
| Complete remission off therapy | Absence of new or established lesions or pruritus while patient is off all BP therapy for at least 2 mo. |
| Mild new activity | <3 lesions/mo that do not heal within 1 wk, or extension of established lesions or pruritus once a wk but less than daily in a patient who has achieved disease control; these lesions have to heal within 2 wk. |
| Relapse/flare | Appearance of ≥3 new lesions/mo or at least 1 large (>10cm diameter) lesion that does not heal within 1 wk, or extension of established lesions or daily pruritus in patient who has achieved disease control. |
| Failure of therapy for initial control | Development of new nontransient lesions or continued extension of old lesions, or failure of established lesions to heal or continued pruritus despite: clobetasol propionate 40g/d for 4 wk; prednisone 0.75mg/kg/d or equivalent for minimum of 3 wk with or without adjuvant therapy; a tetracycline on full dosing for 4 wk. Dapsone 1.5mg/kg/d for 4 wk; methotrexate 15mg/wk (if >60kg and no renal impairment) for 4 wk; azathioprine 2.5 mg/kg/d for 4 wk (if thiopurine s-methyltransferase levels are normal); mycophenolate mofetil 40 mg/kg/d (if normal renal function) for 4 wk. |
Adapted from Murrell et al.24
Topical or systemic corticosteroids are generally the first line of treatment in BP. High-potency topical corticosteroids (Table 2) are particularly indicated in patients with mild or moderate symptoms. In such cases, additional treatment may not be necessary, thereby avoiding the risk of complications due to systemic agents. Very potent topical corticosteroids are also frequently used as adjuncts.
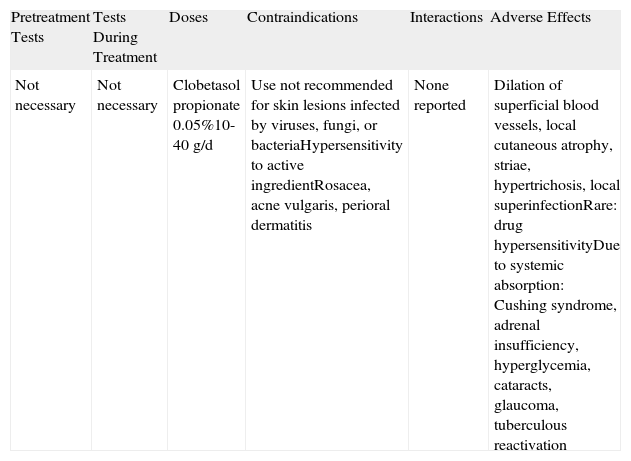
Topical Corticosteroids.
| Pretreatment Tests | Tests During Treatment | Doses | Contraindications | Interactions | Adverse Effects |
| Not necessary | Not necessary | Clobetasol propionate 0.05%10-40g/d | Use not recommended for skin lesions infected by viruses, fungi, or bacteriaHypersensitivity to active ingredientRosacea, acne vulgaris, perioral dermatitis | None reported | Dilation of superficial blood vessels, local cutaneous atrophy, striae, hypertrichosis, local superinfectionRare: drug hypersensitivityDue to systemic absorption: Cushing syndrome, adrenal insufficiency, hyperglycemia, cataracts, glaucoma, tuberculous reactivation |
The effectiveness of topical corticosteroids as monotherapy has been reported in numerous case reports and small series. In a recent study of 96 patients with BP, topical corticosteroids alone (mean dose of 30 g/d of clobetasol propionate 0.05%) achieved disease control in 62% of patients.26 These drugs have also shown better efficacy and safety than systemic corticosteroids in a randomized study.22 The only disadvantage of this randomized study was that it used a rather unconventional treatment regimen, which consisted of 40g per day of clobetasol propionate applied to the entire surface of the body (including healthy skin). The regimen has received considerable criticism, as it is not easy to apply in older patients and it is not known either what quantity of corticosteroids is absorbed into the system. (We have seen patients on this regimen developing Cushing syndrome similar to that seen with systemic corticosteroids.) In a more recent study, the same group described similar efficacy but fewer side effects with lower doses of topical clobetasol.27 Another important consideration with topical corticosteroids is that many patients may not be able to apply the treatment without help. This would obviously make treatment adherence difficult and consequently result in considerably reduced effectiveness.
Systemic CorticosteroidsThe efficacy of systemic corticosteroids (Table 3) has been demonstrated in several studies and is supported by extensive clinical experience. Unless contraindicated, systemic corticosteroids are considered the treatment of choice in patients in whom topical treatment is insufficient or unfeasible. The most common agents used are prednisone and prednisolone.
Systemic Corticosteroids.
| Pretreatment Tests | Tests During Treatment | Doses | Contraindications | Interactions | Adverse Effects |
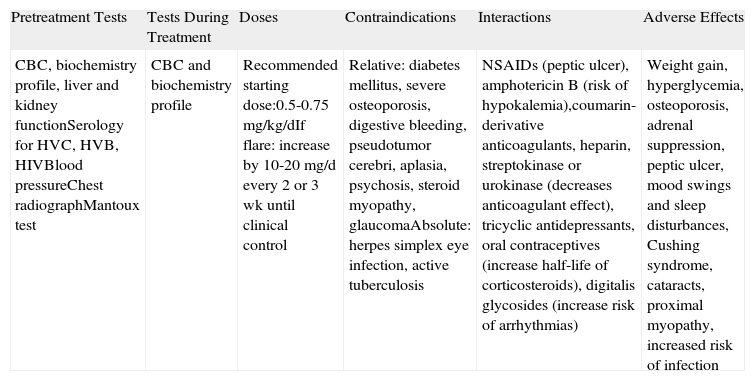
| CBC, biochemistry profile, liver and kidney functionSerology for HVC, HVB, HIVBlood pressureChest radiographMantoux test | CBC and biochemistry profile | Recommended starting dose:0.5-0.75mg/kg/dIf flare: increase by 10-20mg/d every 2 or 3 wk until clinical control | Relative: diabetes mellitus, severe osteoporosis, digestive bleeding, pseudotumor cerebri, aplasia, psychosis, steroid myopathy, glaucomaAbsolute: herpes simplex eye infection, active tuberculosis | NSAIDs (peptic ulcer), amphotericin B (risk of hypokalemia),coumarin-derivative anticoagulants, heparin, streptokinase or urokinase (decreases anticoagulant effect), tricyclic antidepressants, oral contraceptives (increase half-life of corticosteroids), digitalis glycosides (increase risk of arrhythmias) | Weight gain, hyperglycemia, osteoporosis, adrenal suppression, peptic ulcer, mood swings and sleep disturbances, Cushing syndrome, cataracts, proximal myopathy, increased risk of infection |
Abbreviations: CBC, complete blood count; HIV, human immunodeficiency virus; HVB, hepatitis virus B; HVC, hepatitis virus C; NSAIDs, nonsteroidal anti-inflammatory drugs.
The recommended dose ranges from 0.5 to 0.75mg/kg/d, as higher doses have not been seen to improve efficacy and are associated with a higher risk of adverse effects.28 When using doses within the above-mentioned ranges, it is important to administer proton pump inhibitors to prevent gastroduodenal peptic ulcers and also to prescribe calcium supplements, vitamin D, and bisphosphonates from the outset to prevent steroid-induced osteoporosis.
Systemic corticosteroids generally produce favorable outcomes within the first weeks of treatment, enabling subsequent dose tapering. Complete remission is often achieved within 6 to 10 months, after which time these agents can be completely withdrawn.21
Tetracyclines, Erythromycin, and NicotinamideIn patients who respond only partially to corticosteroid therapy or who develop adverse effects, nonimmunosuppressive agents such as tetracyclines, erythromycin, nicotinamide, and sulfone can be used as adjuvant therapy, especially in patients with mild or moderate disease. Thanks to their anti-inflammatory properties, tetracyclines and nicotinamide (Table 4) are used in conditions like BP whose pathogenesis involves immune mechanisms. Furthermore, the fact that these agents do not cause major adverse effects makes them a good choice for older patients and children. Tetracyclines have been seen to inhibit chemotaxis of neutrophils and eosinophils and block the action of certain metalloproteases. They are frequently used in combination with nicotinamide, which acts on different parts of the inflammatory response.
Tetracyclines/Erythromycin/Nicotinamide.
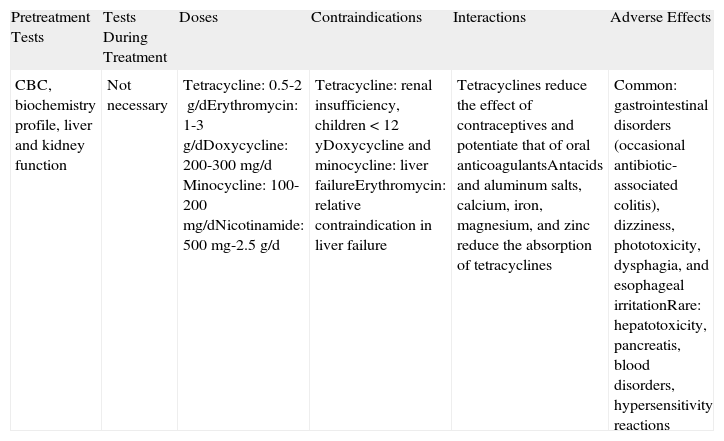
| Pretreatment Tests | Tests During Treatment | Doses | Contraindications | Interactions | Adverse Effects |
| CBC, biochemistry profile, liver and kidney function | Not necessary | Tetracycline: 0.5-2g/dErythromycin: 1-3g/dDoxycycline: 200-300mg/d Minocycline: 100-200mg/dNicotinamide: 500mg-2.5g/d | Tetracycline: renal insufficiency, children <12 yDoxycycline and minocycline: liver failureErythromycin: relative contraindication in liver failure | Tetracyclines reduce the effect of contraceptives and potentiate that of oral anticoagulantsAntacids and aluminum salts, calcium, iron, magnesium, and zinc reduce the absorption of tetracyclines | Common: gastrointestinal disorders (occasional antibiotic-associated colitis), dizziness, phototoxicity, dysphagia, and esophageal irritationRare: hepatotoxicity, pancreatis, blood disorders, hypersensitivity reactions |
Most of the data on the effectiveness of tetracyclines and nicotinamide originate from isolated cases. However, one randomized study of a small group of patients with BP found no significant differences in terms of response to tetracycline 2g/d combined with niacinamide 2g/d versus prednisone therapy alone (40-80g/d).29
SulfoneThe mechanism of action of sulfone (Table 5) has not been fully elucidated, but it appears to involve an anti-inflammatory component, as sulfone is capable of inhibiting neutrophil adherence to vascular endothelial cells, chemotaxis, lipoxygenase production, and the action of neutrophil and eosinophil myeloperoxidase. Its use in BP, either as monotherapy or adjuvant therapy, has not been investigated in randomized trials, but several retrospective studies have reported response rates of between 15% and 45%.30–33 One major drawback of sulfone is that therapeutic doses almost always cause anemia. Reductions in hemoglobin levels of 1 to 2g/dL are common and can sometimes be very poorly tolerated by elderly patients. (We have observed some cases of angina and heart failure in such cases.)
Sulfone.a
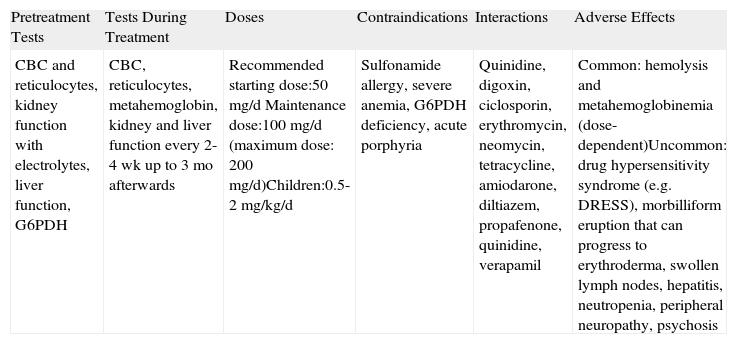
| Pretreatment Tests | Tests During Treatment | Doses | Contraindications | Interactions | Adverse Effects |
| CBC and reticulocytes, kidney function with electrolytes, liver function, G6PDH | CBC, reticulocytes, metahemoglobin, kidney and liver function every 2-4 wk up to 3 mo afterwards | Recommended starting dose:50mg/d Maintenance dose:100mg/d (maximum dose: 200mg/d)Children:0.5-2mg/kg/d | Sulfonamide allergy, severe anemia, G6PDH deficiency, acute porphyria | Quinidine, digoxin, ciclosporin, erythromycin, neomycin, tetracycline, amiodarone, diltiazem, propafenone, quinidine, verapamil | Common: hemolysis and metahemoglobinemia (dose-dependent)Uncommon: drug hypersensitivity syndrome (e.g. DRESS), morbilliform eruption that can progress to erythroderma, swollen lymph nodes, hepatitis, neutropenia, peripheral neuropathy, psychosis |
Abbreviations: CBC, complete blood count; DRESS, drug reaction with eosinophilia and systemic symptoms; G6PDH: glucose-6-phosphate dehydrogenase.
Immunosuppressive agents are indicated for patients with moderate or severe disease in whom high doses of corticosteroids fail to control the disease or in whom effective doses cannot be maintained due to adverse effects.
MethotrexateIn our experience, methotrexate (MTX) (Table 6) is one of the first-line immunosuppressive treatments for BP. It is a folic acid antagonist that is used at low doses as a corticosteroid-sparing agent in numerous diseases. For dermatologists, it has the advantage of being a well-known drug with a very good safety profile that has been used widely in the treatment of psoriasis for decades.
Methotrexate.
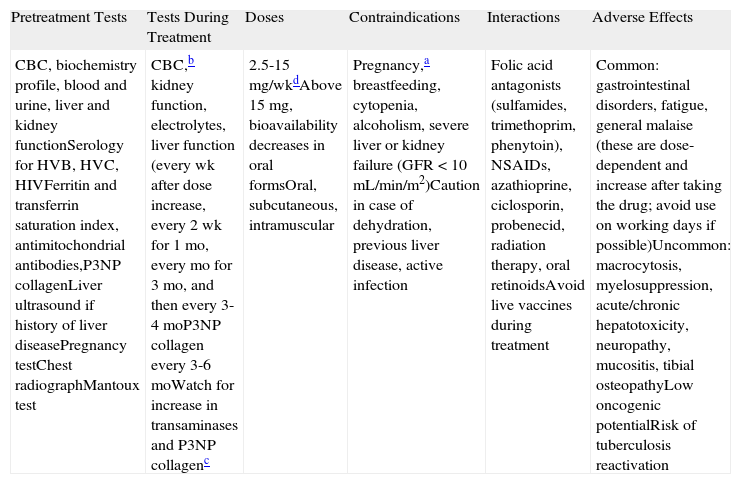
| Pretreatment Tests | Tests During Treatment | Doses | Contraindications | Interactions | Adverse Effects |
| CBC, biochemistry profile, blood and urine, liver and kidney functionSerology for HVB, HVC, HIVFerritin and transferrin saturation index, antimitochondrial antibodies,P3NP collagenLiver ultrasound if history of liver diseasePregnancy testChest radiographMantoux test | CBC,b kidney function, electrolytes, liver function (every wk after dose increase, every 2 wk for 1 mo, every mo for 3 mo, and then every 3-4 moP3NP collagen every 3-6 moWatch for increase in transaminases and P3NP collagenc | 2.5-15mg/wkdAbove 15mg, bioavailability decreases in oral formsOral, subcutaneous, intramuscular | Pregnancy,a breastfeeding, cytopenia, alcoholism, severe liver or kidney failure (GFR <10 mL/min/m2)Caution in case of dehydration, previous liver disease, active infection | Folic acid antagonists (sulfamides, trimethoprim, phenytoin), NSAIDs, azathioprine, ciclosporin, probenecid, radiation therapy, oral retinoidsAvoid live vaccines during treatment | Common: gastrointestinal disorders, fatigue, general malaise (these are dose-dependent and increase after taking the drug; avoid use on working days if possible)Uncommon: macrocytosis, myelosuppression, acute/chronic hepatotoxicity, neuropathy, mucositis, tibial osteopathyLow oncogenic potentialRisk of tuberculosis reactivation |
Abbreviations: CBC, complete blood count; GFR, glomerular filtration rate; HIV, human immunodeficiency virus; HVB, hepatitis B virus; HVC, hepatitis C virus; NSAIDs, nonsteroidal anti-inflammatory drugs; P3NP collagen, type 3 procollagen amino terminal propeptide.
Pregnancy should be avoided for up to 6 mo after finishing treatment. Given its potential effect on spermatogenesis, men should be advised to take measures to prevent pregnancy for at least 3 mo after withdrawal of the drug.
Recommended addition of folic acid (daily) or folinic acid (weekly) to improve gastrointestinal tolerance and reduce toxicity.
If mean corpuscular volume (MCV), increases, administer folic acid supplements. If high MCV (>106fL) persists, withdraw methotrexate.
MTX doses in BP are generally low, ranging from 2.5mg a week (in elderly patients with impaired renal function) to 10mg a week. Higher doses may be administered in some cases, but they should never exceed 15mg a week. It is very important to make clear to the patient (or to his/her carers, as elderly patients are often dependent and living in care) that MTX must be administered in a single weekly dose. There have been reports of cases (in patients with psoriasis) in which a shift to daily doses by health care personnel unfamiliar with MTX resulted in fatal toxic effects.
The effectiveness of MTX in BP is supported by evidence from numerous case reports and series in which it has been used in monotherapy as a systemic corticosteroid-sparing drug in patients with moderate to severe disease, or in association with topical corticosteroids in patients with less severe disease.34,35 In one prospective study involving 11 patients administered MTX and topical corticosteroids, all the patients responded rapidly.34 The authors recommended starting at a dose of 5mg/wk and increasing this by 2.5 mg every week (up to a maximum of 12.5mg/wk) until clinical remission is achieved.
More powerful immunosuppressive drugs such as azathioprine (AZA), mycophenolate, and cyclophosphamide are indicated for severe or refractory cases of BP.
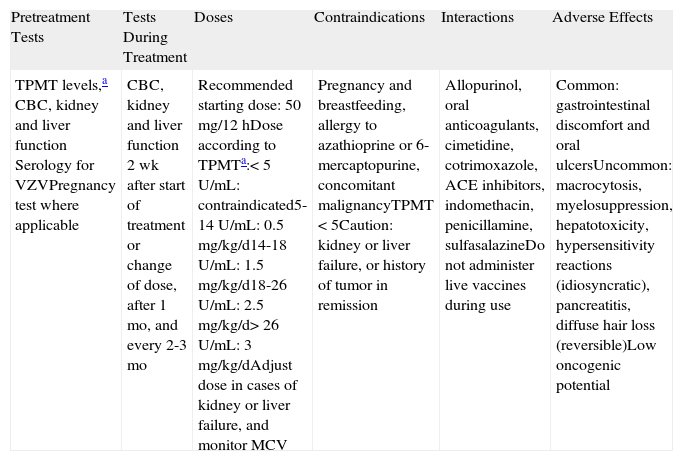
AzathioprineAZA (Table 7) is an imidazolyl derivative of 6-mercaptopurine with anti-inflammatory and immunosuppressive properties. It is administered orally or intravenously. To date, no significant differences in response have been observed in BP between patients treated with AZA as an adjunct agent and patients treated with systemic corticosteroids alone.36,37 There is, however, extensive experience with the use of AZA as a corticosteroid-sparing drug, and in our setting it is the most common drug used for this purpose.
Azathioprine.
| Pretreatment Tests | Tests During Treatment | Doses | Contraindications | Interactions | Adverse Effects |
| TPMT levels,a CBC, kidney and liver function Serology for VZVPregnancy test where applicable | CBC, kidney and liver function 2 wk after start of treatment or change of dose, after 1 mo, and every 2-3 mo | Recommended starting dose: 50mg/12hDose according to TPMTa:<5U/mL: contraindicated5-14U/mL: 0.5mg/kg/d14-18U/mL: 1.5 mg/kg/d18-26U/mL: 2.5 mg/kg/d> 26U/mL: 3mg/kg/dAdjust dose in cases of kidney or liver failure, and monitor MCV | Pregnancy and breastfeeding, allergy to azathioprine or 6-mercaptopurine, concomitant malignancyTPMT <5Caution: kidney or liver failure, or history of tumor in remission | Allopurinol, oral anticoagulants, cimetidine, cotrimoxazole, ACE inhibitors, indomethacin, penicillamine, sulfasalazineDo not administer live vaccines during use | Common: gastrointestinal discomfort and oral ulcersUncommon: macrocytosis, myelosuppression, hepatotoxicity, hypersensitivity reactions (idiosyncratic), pancreatitis, diffuse hair loss (reversible)Low oncogenic potential |
Abbreviations: ACE, angiotensin-converting enzyme; CBC, complete blood count; MCV, mean corpuscular volume; TPMT, thiopurine S-methyltransferase; VZV, varicella-zoster virus.
In our setting 0.3% of the population have low TPMT activity and 11.9% have moderate activity. These patients can develop severe adverse effects when taking azathioprine, such as cholestatic hepatitis, severe myelosuppression, pancreatitis, or gastric toxicity. Patients with high TPMT activity (>80% of the population) may need high doses of azathioprine to achieve an effect, and these tend to carry a higher risk of hepatotoxicity.
The therapeutic effect is normally achieved within 6 to 10 weeks of commencement of treatment. It is important to measure TPMT enzyme levels before prescribing AZA. TPMT metabolizes this drug and therefore knowledge of its activity helps to prevent hematologic toxicity through dose adjustments, although it does not provide information on hepatic toxicity. TPMT activity depends on 3 mutant alleles of the TPMT gene—TPMT*2, TPMT*3A, and TPMT*3C—which are involved in 95% of cases of low or moderate enzyme activity. The presence of these 3 alleles is predictive of phenotype. Heterozygous patients (with 1 wild-type allele and 1 mutant allele) will have moderate TPMT activity, while homozygous patients (with 2 mutant alleles) will have complete TPMT deficiency (this is the case of approximately 1 in every 300 members of the general population). TPMT activity can be measured by 2 methods: determination of the intraerythrocytic activity of the enzyme (a less accurate method) or allele identification by polymerase chain reaction.
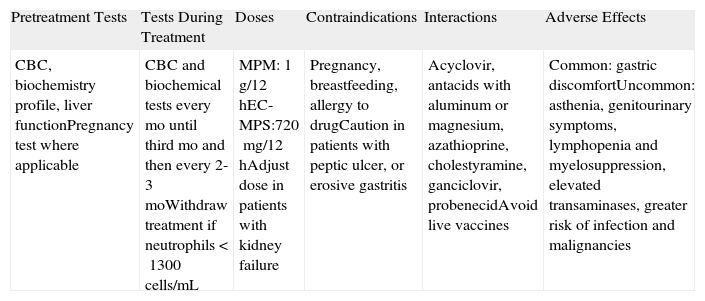
MycophenolateMycophenolate (Table 8) inhibits the synthesis of DNA nucleotides. Two different drugs are used in clinical practice: mycophenolate mofetil (MPM) and enteric-coated mycophenolate sodium (EC-MPS). MPM is a prodrug of mycophenolic acid that has been used since 1997 for the prophylaxis of kidney transplant rejection. According to its summary of product characteristics, it is now also indicated for liver and heart transplantation. EC-MPS was designed to reduce the gastrointestinal intolerance frequently seen with MPM. The enteric coating delays the release of the active substance, preventing contact with the acid medium of the stomach. EC-MPS is currently licensed by the US Food and Drug Administration (FDA) for use in kidney transplantation.
Mycophenolate Mofetil (MPM) and Enteric-Coated Mycophenolate Sodium (EC-MPS).
| Pretreatment Tests | Tests During Treatment | Doses | Contraindications | Interactions | Adverse Effects |
| CBC, biochemistry profile, liver functionPregnancy test where applicable | CBC and biochemical tests every mo until third mo and then every 2-3 moWithdraw treatment if neutrophils <1300cells/mL | MPM: 1g/12hEC-MPS:720mg/12hAdjust dose in patients with kidney failure | Pregnancy, breastfeeding, allergy to drugCaution in patients with peptic ulcer, or erosive gastritis | Acyclovir, antacids with aluminum or magnesium, azathioprine, cholestyramine, ganciclovir, probenecidAvoid live vaccines | Common: gastric discomfortUncommon: asthenia, genitourinary symptoms, lymphopenia and myelosuppression, elevated transaminases, greater risk of infection and malignancies |
Abbreviation: CBC, complete blood count.
Few studies to date have analyzed the use of MPM (or EC-MPS) in the treatment of BP. It is generally used in a similar fashion to AZA, i.e., as a corticosteroid-sparing agent, although it has also produced favorable results when used as monotherapy.38 It is as effective as AZA and has a better safety profile, but it is considerably more expensive. In a recent study of patients with BP, corticosteroids combined with MPM showed similar efficacy to corticosteroids combined with AZA.39
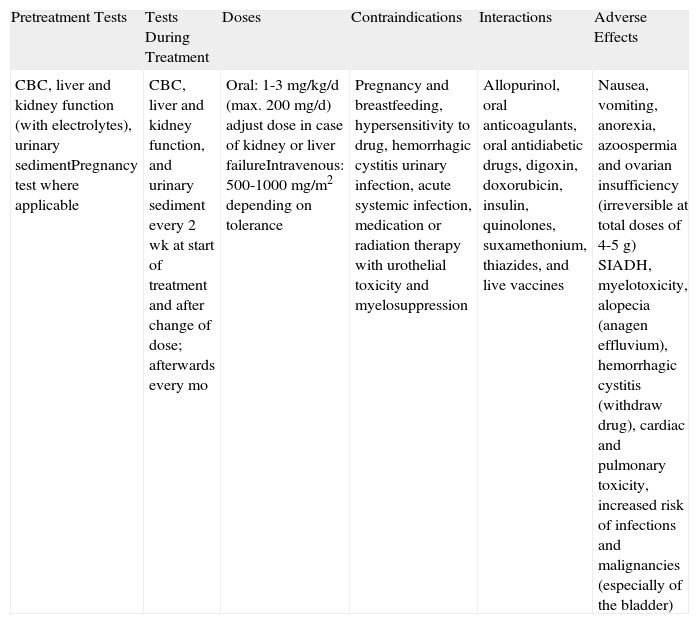
CyclophosphamideThe toxicity of cyclophosphamide (Table 9), particularly its effects on fertility, myelosuppression, and increased risk of malignancy, is an important limitation to its use in patients in whom other drugs are contraindicated or ineffective. Cyclophosphamide is one of the most potent suppressors of cell-mediated and humoral immunity, but it is also one of the most effective drugs of its kind. It is an alkylating agent, administered orally or intravenously, that is metabolized by cytochrome P450. Although no randomized trials have investigated its value in BP, several case reports and small series have reported on its use as an adjuvant, corticosteroid-sparing agent. The results have shown that it is a potent drug but that it is considerably more toxic than other immunosuppressants.40,41 In our experience, cyclophosphamide can be a very good second-line option when used at low doses (50-100mg/d), particularly in elderly patients in whom long-term side effects are perhaps not the overriding concern.18
Cyclophosphamide.
| Pretreatment Tests | Tests During Treatment | Doses | Contraindications | Interactions | Adverse Effects |
| CBC, liver and kidney function (with electrolytes), urinary sedimentPregnancy test where applicable | CBC, liver and kidney function, and urinary sediment every 2 wk at start of treatment and after change of dose; afterwards every mo | Oral: 1-3mg/kg/d (max. 200mg/d) adjust dose in case of kidney or liver failureIntravenous: 500-1000mg/m2 depending on tolerance | Pregnancy and breastfeeding, hypersensitivity to drug, hemorrhagic cystitis urinary infection, acute systemic infection, medication or radiation therapy with urothelial toxicity and myelosuppression | Allopurinol, oral anticoagulants, oral antidiabetic drugs, digoxin, doxorubicin, insulin, quinolones, suxamethonium, thiazides, and live vaccines | Nausea, vomiting, anorexia, azoospermia and ovarian insufficiency (irreversible at total doses of 4-5g) SIADH, myelotoxicity, alopecia (anagen effluvium), hemorrhagic cystitis (withdraw drug), cardiac and pulmonary toxicity, increased risk of infections and malignancies (especially of the bladder) |
Abbreviations: CBC, complete blood count; SIADH, syndrome of inappropriate antidiuretic hormone secretion.
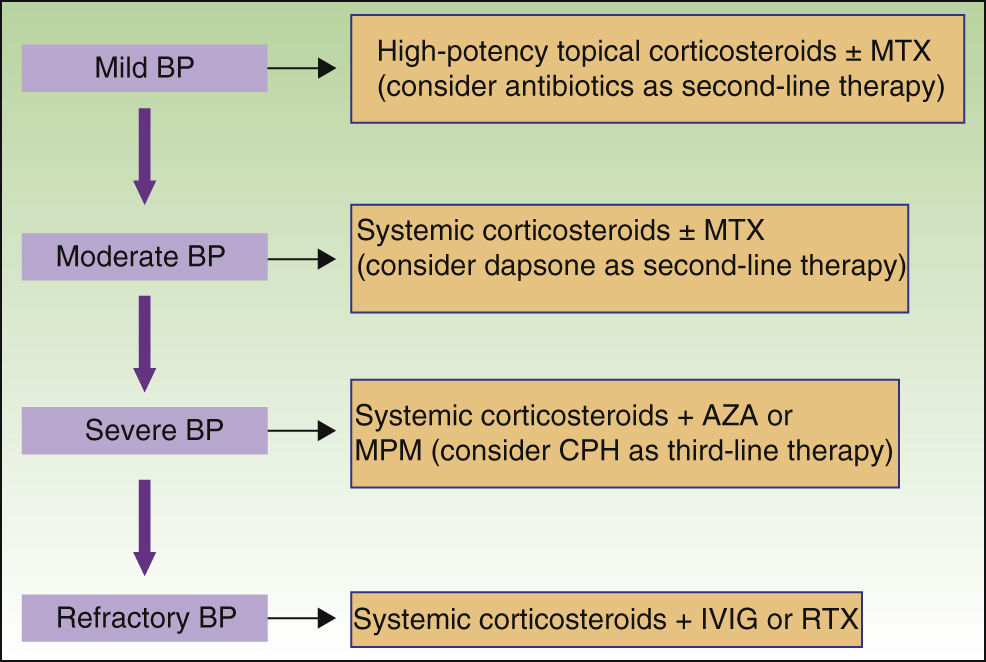
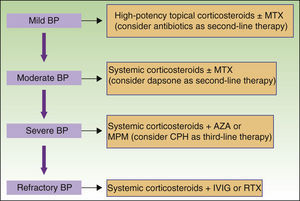
New nonimmunosuppressive treatments, with different mechanisms of action, have emerged in recent years. Examples are IVIG therapy and rituximab, which have been used as third-line alternatives, particularly in cases of severe or refractory BP (Fig. 8).
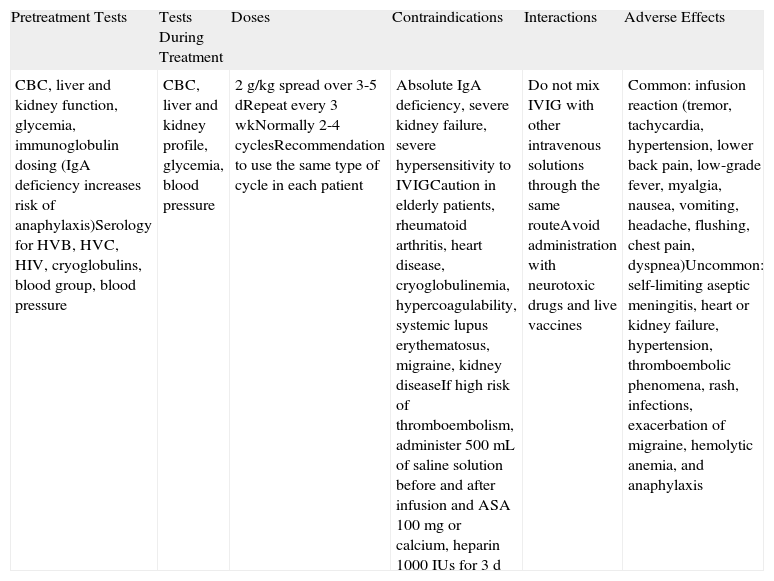
Intravenous ImmunoglobulinsThe immunoglobulins used in IVIG therapy (Table 10) are obtained from the plasma of numerous donors. Approximately 90% of the immunoglobulins present in each formula are IgG and the remainder are IgE and IgM. The mechanism of action involved is complex and not yet fully understood. IVIG therapy has been associated with a clear, rapid decrease in levels of pathogenic antibodies in several autoimmune diseases. This decrease has also been demonstrated in animal models42 and anti-basement membrane antibodies in BP.
Intravenous Immunoglobulin (IVIG) Therapy.a
| Pretreatment Tests | Tests During Treatment | Doses | Contraindications | Interactions | Adverse Effects |
| CBC, liver and kidney function, glycemia, immunoglobulin dosing (IgA deficiency increases risk of anaphylaxis)Serology for HVB, HVC, HIV, cryoglobulins, blood group, blood pressure | CBC, liver and kidney profile, glycemia, blood pressure | 2g/kg spread over 3-5 dRepeat every 3 wkNormally 2-4 cyclesRecommendation to use the same type of cycle in each patient | Absolute IgA deficiency, severe kidney failure, severe hypersensitivity to IVIGCaution in elderly patients, rheumatoid arthritis, heart disease, cryoglobulinemia, hypercoagulability, systemic lupus erythematosus, migraine, kidney diseaseIf high risk of thromboembolism, administer 500mL of saline solution before and after infusion and ASA 100mg or calcium, heparin 1000IUs for 3 d | Do not mix IVIG with other intravenous solutions through the same routeAvoid administration with neurotoxic drugs and live vaccines | Common: infusion reaction (tremor, tachycardia, hypertension, lower back pain, low-grade fever, myalgia, nausea, vomiting, headache, flushing, chest pain, dyspnea)Uncommon: self-limiting aseptic meningitis, heart or kidney failure, hypertension, thromboembolic phenomena, rash, infections, exacerbation of migraine, hemolytic anemia, and anaphylaxis |
Abbreviations: ASA, acetylsalicylic acid; CBC, complete blood count; HIV, human immunodeficiency virus; HVB, hepatitis virus B, HVC, hepatitis virus C; IgA, immunoglobulin A.
The infusion should be slow (approximately 6h, starting with 15mL/min for 15min). Premedication with dexchlorpheniramine and paracetamol 1h before start of infusion is recommended. The infusion rate should be reduced if the patient experiences chest tightness, rubeosis, or hypotension during infusion. If the symptoms do not improve, intravenous dexchlorpheniramine, corticosteroids, and even adrenaline may need to be administered. Infusion must be stopped in the event of a severe reaction.
While BP is not among the FDA-approved indications for IVIG therapy, there have been numerous reports of it being used successfully to treat this disorder. Most of the patients, including 15 from a prospective study,43 had refractory BP that failed to respond to other treatments but responded adequately to IVIG therapy. The addition of immunosuppressive drugs has been seen to reduce the rebound effect seen following the rapid reduction of pathogenic antibodies induced by IVIG therapy.
IVIG therapy is expensive, fast-acting, and nonimmunosuppressive, and must be administered in an inpatient or day-hospital setting. Because of its cost, it would only be indicated for patients who do not respond to or who develop adverse effects to second-line treatments, or for patients in whom a rapid response is required and immunosuppression is no longer an option.
RituximabRituximab (RTX) (Table 11) is a humanized chimeric monoclonal antibody that selectively targets CD20 cells. These include pre-B cells and mature B cells, but not stem or plasma cells. The drug was initially developed to control lymphoproliferative B-cell disorders, but in recent years, used either alone or in combination with IVIG therapy, it has proven to be effective at controlling an increasing number of autoimmune diseases that do not respond to other treatments.
Rituximab.a
| Pretreatment Tests | Tests During Treatment | Doses | Contraindications | Interactions | Adverse Effects |
| CBC, B-cell count, serology for HVB, HVC, HIVMantoux test (PPD) and chest radiographConsider pneumococcal vaccination | CBC and B-cell count | Most common dose: 375mg/m2/wk for 4 wkConsider single administration in case of flare | Active hepatitis B or C infection, hypersensitivity to murine proteins, uncontrolled active infection, HIV infection, with CD4+ T-cell count <250cells/μL, severe heart failurePregnancy and breastfeeding (contraception recommended for up to a year after finishing treatment) | Avoid attenuated vaccines and anti-TNF agents (up to 16 wk after last dose) | Common: Infusion reactionUncommon: hypotension, urticaria-like rash/angioedema, bronchospasm, thrombopenia (normally after first infusion, reversible), increased risk of infections, toxic epidermal necrolysis, progressive multifocal leukoencephalopathy, arrhythmias, heart failure |
Abbreviations: CBC, complete blood count; HIV, human immumodeficiency virus; HVB, hepatitis virus B, HVC, hepatitis virus C; PPD, tuberculin purified protein derivative; TNF, tumor necrosis factor.
Must be administered slowly (5-6h for first infusion). Premedication with antihistamines and intravenous corticosteroids is recommended (generally only before first infusion). Some authors recommend infusing with intravenous immunoglobulin at a single dose of 400mg/kg the day before rituximab infusion to prevent infections.
The effects of RTX take approximately a month to appear and are maintained for 9 to 12 months (the time it takes for B cells to recover). It has proven effective in autoimmune blistering disorders with higher morbidity and mortality than BP, such as pemphigus vulgaris and pemphigus foliaceous. Consideration of rituximab in refractory cases of BP is warranted considering the involvement of B cells in the pathophysiology of this disease. However, experience with its use in this setting is still limited to isolated cases and no randomized trials have investigated factors such as dose regimens, monitoring, etc.
In a recent retrospective study in which 5 patients with BP who had not responded to several drugs were treated with RTX, 3 of the patients showed complete response and a fourth showed partial response. The other patient, who had heart disease, died unexpectedly several days after starting treatment.44
RTX must be administered in an inpatient or day-hospital setting. Its toxicity and associated mortality are not negligible, particularly in relation to the risk of serious opportunistic or other infections. It should therefore only be considered in patients with severe disease who do not respond to conventional treatments.45
Therapeutic Plasma ExchangeTherapeutic plasma exchange is a costly technique that is not available in most hospitals. It is therefore not widely used in our setting, even for autoimmune blistering disorders with higher morbidity than BP. The technique involves substituting a considerable volume of the patient's blood (2-5L) with replacement plasma.
Two randomized studies have compared the effects of plasma exchange combined with systemic corticosteroids versus corticosteroids alone36 or in combination with AZA.46 No significant differences were observed between plasma exchange and AZA as adjuncts, although in one of the studies46 a reduction was observed in the dose of corticosteroids in the plasma exchange group.
Therapeutic plasma exchange increases the risk of thrombosis, infections, and electrolyte abnormalities. Furthermore, it requires the use of an arteriovenous fistula, which carries an addition risk of adverse effects. It is generally administered in association with systemic corticosteroids or immunosuppressive drugs, which help to reduce the rebound effect produced by the activation of autoreactive lymphocytes following the exchange.
Plasma exchange is thus an exceptional treatment that is rarely used in BP. It should be reserved for patients with acute, severe disease who have high levels of circulating antibodies and contraindications for other treatments.
Evidence-Based ConclusionsThe latest update on interventions for BP published in The Cochrane library in 2010 included 10 randomized controlled studies of the treatment of BP.47 The following conclusions were drawn:
- 1.
Very potent topical corticosteroids are effective and presumably safe.
- 2.
Starting doses of prednisolone of over 0.75mg/kg/d have not been demonstrated to improve clinical response.
- 3.
The efficacy of plasma exchange or AZA as adjuncts to systemic corticosteroids has not been demonstrated.
- 4.
No differences have been found between the efficacy of AZA and mycophenolate mofetil as adjuncts to systemic corticosteroids.
- 5.
Combination treatment with tetracyclines and nicotinamide appears to be effective, although studies are needed to confirm this.
The authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Fuertes de Vega I, Iranzo-Fernández P, Mascaró-Galy JM. Penfigoide ampolloso: guía de manejo práctico. Actas Dermosifiliogr. 2014;105:328–346.