Benign cephalic histiocytosis (BCH) is a rare type of non-Langerhans cell histiocytosis (NLCH) of unknown etiology; it affects children under 3 years of age and tends to manifest in the first year of life.1 Clinical characteristics include the appearance of multiple maculopapular lesions measuring between 2 and 8 mm in diameter, with a yellowish-red or brownish-red color, mainly distributed on the head and neck.2
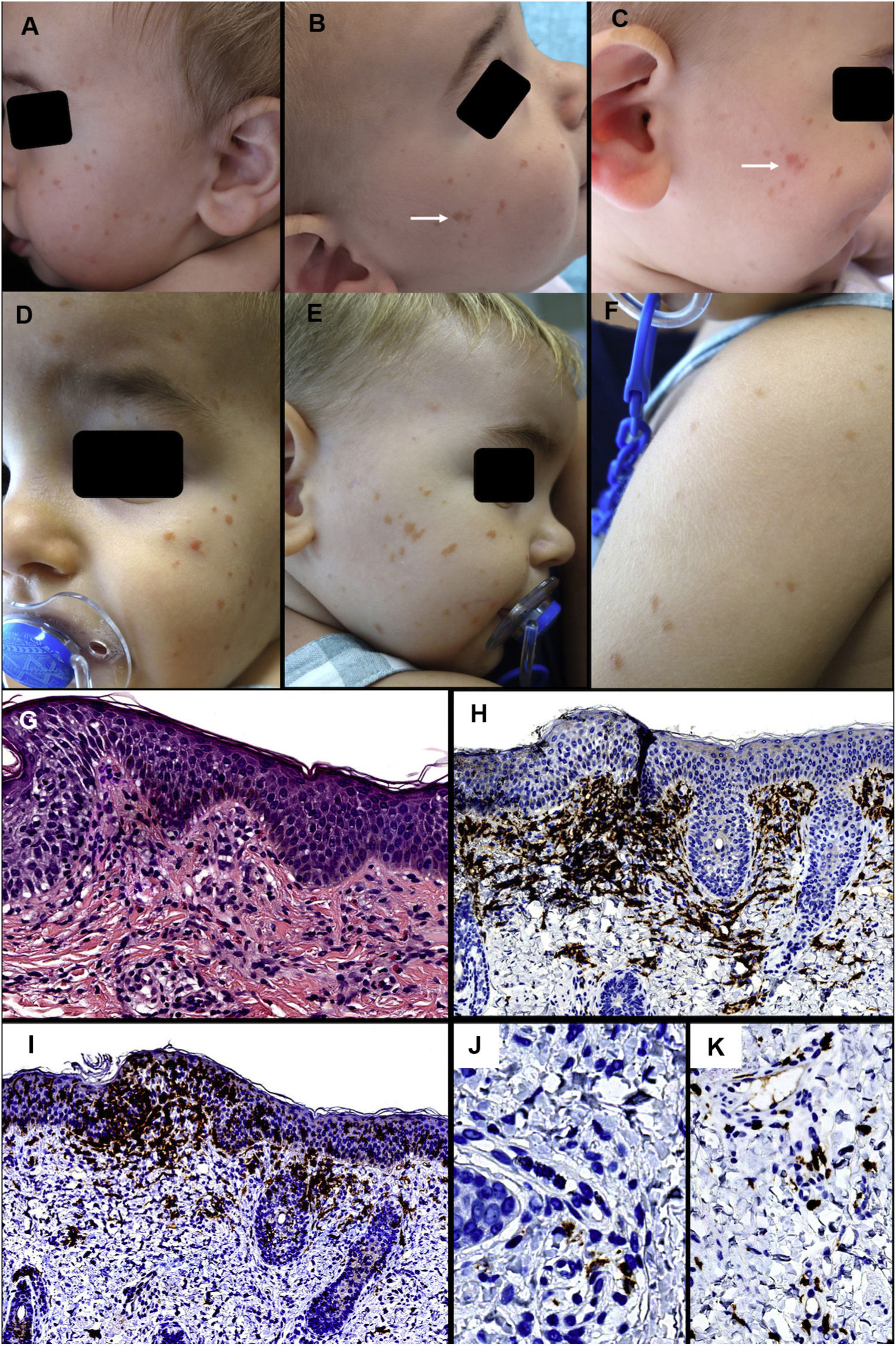
Case 1: A 7-month-old infant with no relevant past history was evaluated for brownish maculas on the face, measuring between 2 and 4 mm in diameter, which had appeared 3 months earlier (Figs. 1A and 1B). The mother reported no other symptoms, except for a change in color of the lesions on rubbing (Fig. 1B and 1C). A follow-up visit found an increase in number and intensity of the lesions, and the appearance of new elements on the upper part of the back and the arms (Fig. 1D-1F). A skin biopsy revealed a dense inflammatory infiltrate in the superficial dermis, consisting predominantly of macrophages, with few eosinophils. Immune staining was positive for CD163 and CD68 in most cells. Fifteen percent of cells were positive for CD1a and negative for S100 and langerin in the dermal infiltrate, and intraepidermal levels were also very high. Furthermore, the c-KIT marker revealed a discrete increase in the predominantly perivascular mast-cell count (Fig. 1G-1 K). Additional tests (abdominal ultrasound, blood test and urine sediment) revealed a reduction in hemoglobin (10.1 g/dL) and a discrete increase in the size of the spleen; for this reason, the patient was sent to the pediatric hematologist for evaluation.
First patient. Clinical presentation: A, Multiple brownish-red papules on the left cheek. B and C, Lesions on the right cheek that took on a more erythematous and edematous appearance on rubbing. D-F, Clinical deterioration of the lesions on the facial area and appearance of lesions on the upper back and arms.
Histology: G, Discrete mononuclear infiltrate in the superficial dermis with disperse eosinophils. H, The vast majority of infiltrate cells are positive for CD163. I, Numerous intraepidermal and dermal cells positive for CD1a. J, Many of these are negative for langerin. K, A discrete increase in perivascular mast cells can be observed. (G, HE × 200; H, CD163 × 100; I, CD1a ×100; J, langerin ×400; K, c-kit ×200).
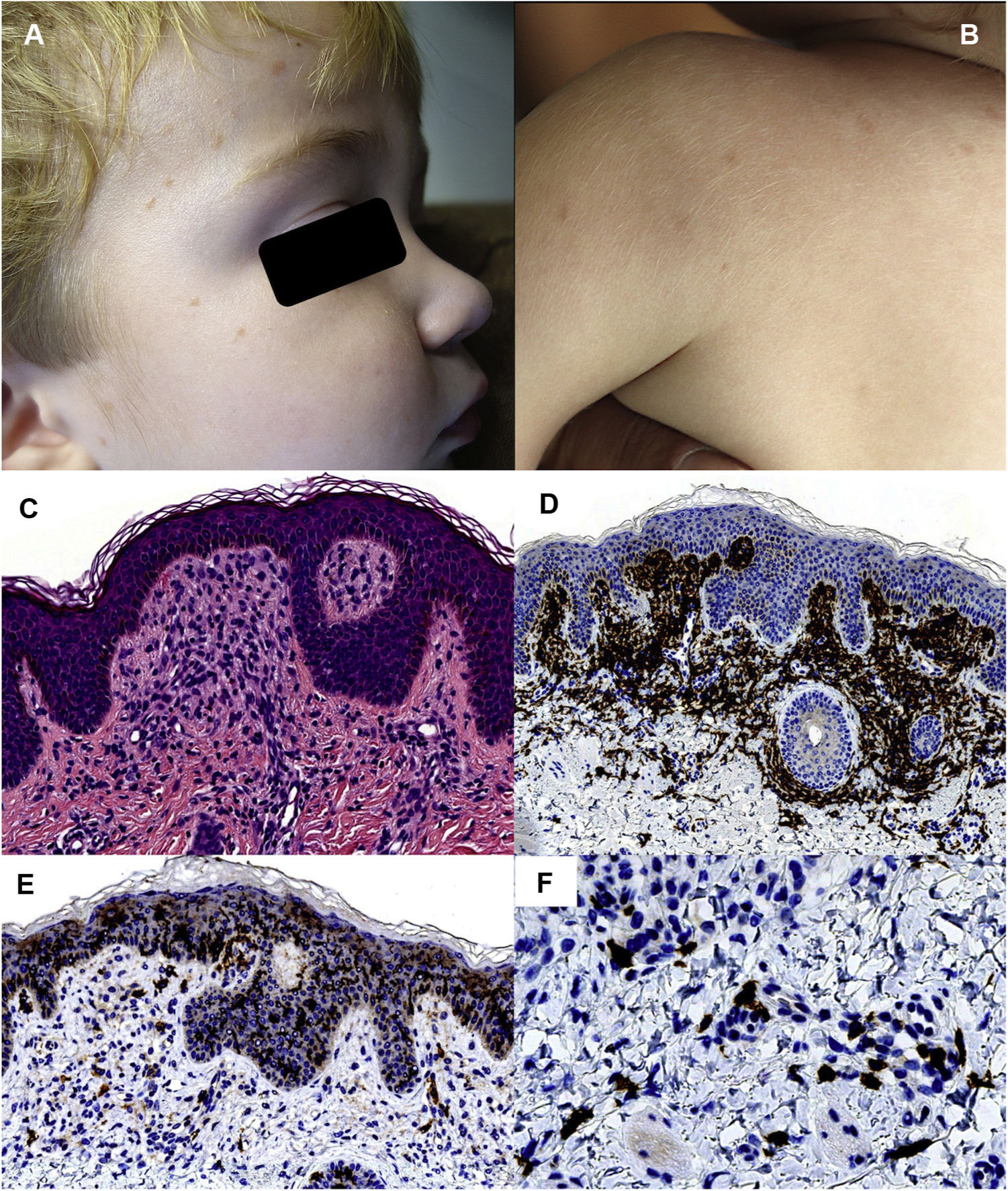
Case 2: A 1-year-old child with no past history of interest visited with yellowish-red maculopapular lesions that had appeared on the face and upper part of the torso 2 months earlier (Figs. 2A and 2B). The lesions took on a redder color on crying. Histopathology revealed a mononuclear inflammatory infiltrate in the superficial dermis. Immune staining of the infiltrate was positive for CD163 and CD68 and negative for CD1a, S100, and langerin (Fig. 2C-2F). As in the previous case, a discrete increase in the number of mast cells was observed. Blood tests revealed an elevated white blood cell count (20450/L) with no other infection data; the patient was therefore referred to the pediatric hematologist for evaluation.
Second patient. Clinical presentation: A and B, Brownish-red maculopapular lesions on the facial area, upper back, and arms. Histology: C, Mononuclear infiltrate in the superficial dermis. D, The dermal population is mostly positive for CD163. E, An unusually high number of cells positive for CD1a is not observed. F, A slight increase in perivascular mast cells can be observed. (D, HE × 200; E, CD163 × 100; F, CD1a ×100; G, c-kit ×400).
A diagnosis of iron deficiency anemia was made in the pediatric hematology consultation in the first case and blood-test and ultrasound results were normal a few months later. The white blood cell count was normal in follow-up visits in the second patient. Given the epidemiologic clinical context and after ruling out systemic involvement, both children were diagnosed with BCH.
Non-Langerhans cell histiocytoses located in the skin and mucosa include a variety of entities that are classified depending on clinical signs and symptoms, immunophenotype, and the presence or absence of systemic involvement.1 According to a recently published classification, BCH belongs to the group of juvenile xanthogranulomas (in group C of non-Langerhans cell histiocytoses), together with other entities that do not have systemic involvement.1 The term cephalic is subject to debate, as involvement of the torso and upper extremities has been observed in a high percentage of cases.3 Histologically, it is characterized by a histiocyte infiltrate in the superficial and middle dermis, with positive staining for CD68 and CD163, and negative staining for CD1a, S100, and langerin.2,3 Cases of BCH have been published, however, with immunophenotypes positive for S1004 and CD1a.5 Darier’s sign is generally negative in histiocytoses, although it has presented in some cases.6 In our 2 children, the lesions took on a more erythematous tone in certain situations. This “pseudo-Darier” phenomenon may be due, in histologic terms, to the discrete increase in mast cells observed in both biopsies (Figs. 1K and 2G). And while cases of cutaneous mastocytosis with mast-cell infiltrates positive for CD1a exist,7 in our first case, the mast cells observed did not correspond to those of the predominant histiocyte infiltrate (CD1a positive), as in the second case. The differential diagnosis of BCH includes entities such as urticaria pigmentosa, lichenoid sarcoidosis, and other types of histiocytosis.2,3 A histologic overlap is thought to exist between BCH and other types of non-Langerhans cell histiocytoses, such as juvenile xanthogranuloma and generalized eruptive histiocytosis.1,3 For this reason, a correct diagnosis requires taking into account the clinical and epidemiologic characteristics and the existence of extracutaneous involvement.1 In the first case, the immunophenotypic findings led us to consider a diagnosis of indeterminate cell histiocytosis; however, this entity tends to affect adult patients and the lesions are more frequently located on the torso.1 In our 2 children, the young age of presentation, the distribution of the lesions, the favorable clinical outcome with attenuation of the lesions in the following months, and the lack of systemic involvement led us to a final diagnosis of BCH.
BCH is a rare entity, but prognosis is good and it is self-limiting in most cases.1–3 Although it is not generally associated with other diseases, cases of association with diabetes mellitus8 and diabetes insipidus9 have been reported. Given the few cases of BCH published in the literature, either due to its low frequency or underdiagnosis, we believe that these 2 new cases with unusual immunophenotypic findings may provide new data that can help to characterize the entity histologically.
Please cite this article as: Silva Díaz E, Vázquez Fernández B, Monteagudo Castro C, Martín Hernández JM. Histiocitosis cefálica benigna simulando una mastocitosis. Actas Dermosifiliogr. 2022;113:195–198.
This work was presented in poster format at the 30th Meeting of the Spanish Group of Pediatric Dermatology GEDP held in Bilbao on January 25 and 26, 2019.