Psoriasis is a chronic and recurrent skin disease, affecting up to 2% of the population. Biological therapy has revolutionized the management of this disease. Guselkumab and risankizumab are monoclonal antibodies anti-interleukin (IL)-23 approved for treatment of severe moderate plaque psoriasis.
Multiples studies that support the effectiveness of these treatments in real-life settings have been published. However, real-life data concerning the outcomes of psoriatic patients treated with anti-IL-23 drugs, after failing anti-IL-17 regimens are limited.
We present a retrospective study with 20 patients who had previously failed anti IL17 therapy treated with guselkumab or risankizumab during one year in clinical practice.
We analyze the demographic characteristics of patients, previous systemic treatments, and psoriasis severity using Psoriasis Area and Severity Index (PASI). PASI during the follow-up at weeks 4, 16, 28, and 52 were used to evaluate the response to the treatment. In addition, adverse events (AEs) were evaluated.
A descriptive analysis was carried out and the qualitative and quantitative variables were analyzed with chi-squared and T tests respectively considering p value<.05 statistically significant.
Overall, 20 patients were included. Demographic characteristics are shown in Table 1.
Demographic characteristics of patients.
| Study population | |
| Patients, n | 20 |
| Age, years | 48.90 (11.17) |
| Sex | |
| Female | 7 (35%) |
| Male | 13 (65%) |
| Psoriasis duration, years | 23.95 (9.44) |
| BMI, mean | 30.85 (11.52) |
| Obesity (BMI>30) | 13 (64%) |
| Special locations | |
| Scalp | 5/20 (25% |
| Nails | 2/20 (10%) |
| Palmo-plantar | 2 (10%) |
| Comorbidities | 17/20 (85%) |
| Diabetes | 3/20 (15%) |
| Hypertension | 10/20 (50%) |
| Dyslipidaemia | 10/20 (50%) |
| Psoriatic arthritis | 1/20 (5%) |
| Fatty liver | 7/20 (35%) |
| Latent tuberculosis infection | 7/20 (35%) |
| Previous conventional systemic treatments, n (%) | |
| Phototherapy | 11/20 (55%) |
| Methotrexate | 17/20 (85%) |
| Ciclosporin | 17/20 (85%) |
| Acitretin | 13/20 (65%) |
| Apremilast | 6/20 (30%) |
| Previous biologics therapy mean | 4 (1.75) |
| Number of previous biological treatments | |
| 1 | 2/20 (10%) |
| 2 | 3/20 (15%) |
| 3 | 2/20 (10%) |
| ≥4 | 13/20 (65%) |
| AntiIL17 therapy | |
| Ixekizumab | 15/20 (75%) |
| Secukinumab | 4/20 (20%) |
| Brodalumab | 1/20 (5%) |
| Study therapies n (%) | |
| Guselkumab | 11/20 (55%) |
| Risankizumab | 9/20 (45%) |
| Score baseline | |
| PASI | 9.89 (6.79) |
| BSA | 20.31 (23.43) |
| DLQI | 9.33 (5.93) |
| Adverse events n (%) | 3 (15%) |
| Pain at the injection site | 1 (5%) |
| Mild low back pain | 1 (5%) |
| Arthralgias | 1 (5%) |
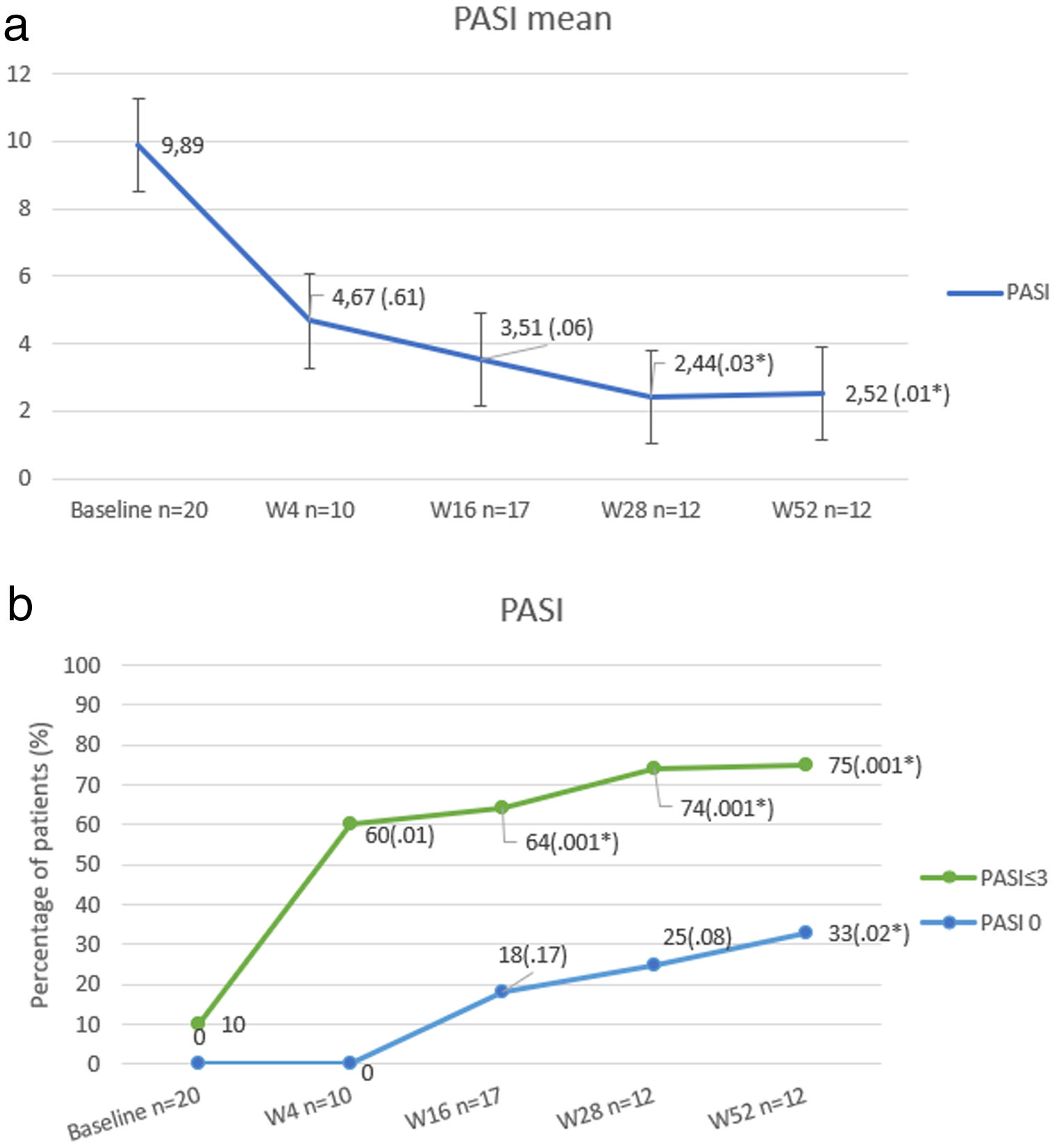
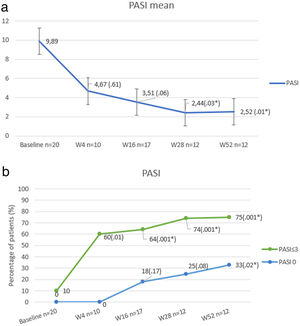
At baseline, mean PASI score was 9.89 (±6.79), which was reduced up to 2.52 (±2.99) at week 52 (P<.01). The percentage of patients who achieved PASI 0 and PASI≤3 is shown in Fig. 1.
No significant differences were found between the improvements in patients with risankizumab and guselkumab (p=0.8).
In 3 patients, adverse effects were reported, all of them mild. One patient showed arthralgias at week 28, which caused the discontinuation of the treatment with risankizumab.
Treatment with antiIL23 was stopped in 3 patients (15%), in 2 cases (one patient with risankizumab and one with guselkumab) (10%) due to a primary inefficacy (week 28), and the remainder due to the reported adverse effect (arthralgias).
Our results show that the change of therapeutic target in patients who have failed anti IL17 regimens for an anti IL23 biologic may be an effective therapeutic alternative in a high percentage of cases. In other real-life series,2 PASI≤3 has been considered as the primary outcome, as it is related to significant improvement in patients’ quality of life. PASI≤3 at week 52 was reached in the majority (75%), although most of the patients had failure to respond to multiple previous biological therapies (average of 4).
No statistically significant difference or predicting factor of response was found between patients who achieved PASI≤3 compared to non-responder patients at week 28. However, it was observed that patients who did not reach PASI≤3 at week 52, had a higher BMI (39.52 vs 29.86; p=0.041) and presented more frequently Diabetes (67% vs 0%; p=0.007).
Mean PASI of patients who had not received previous treatment with ustekinumab (33%) was slightly lower at week 52 compared with those who were treated in the past with anti Il12-23 therapy, (1.35 vs 3.05) without significant differences (p=0.38). Neither the number of previous therapies nor the previous administration of any specific biological treatment implied any statistically significant change in the response to treatment with anit-IL23.
In the literature there are multiple studies that have shown in real life3,4 that anti IL23 drugs were very effective treatments, with results comparable to pivotal clinical trials of these drugs.5,6
Nevertheless, real-life data of patients treated with new anti-interleukin (IL)-23 drugs, after failing anti-IL-17 regimens are scarce.
Bonifati et al.1 reported a series with 12 patients prospectively studied for 6 months. The demographic characteristics were similar to our own, excepting baseline average PASI, which was lower (6.25 (±7.80)), and they showed statistically significant improvements in PASI at both 3 and 6 months.
Megna et al.7 studied 8 patients retrospectively with previous failure to anti IL17 and/or ustekinumab achieving a reduction in absolute PASI from 11.9 to 5.5 at week 4 and 3.3 at week 16, data similar to our series.
Megna et al.8 have just reported a study with 44 patients to evaluate the long-term efficacy of guselkumab in patients who previously failed anti-IL17. They have described a significant improvement, achieving a reduction in absolute PASI from 13.9 to 0.9 at week 52.
Our study displays several limitations. The number of patients is limited and due to its retrospective design, the patients are not homogeneous in terms of several baseline characteristics, which renders the extrapolation of results difficult.
We present a series of psoriatic patients treated with anti IL23 in real-life after failure to treatment with anti IL17. The change of therapeutic target proved an effective and safe alternative in a high number of patients in clinical practice, despite them being of high complexity.
Conflict of interestsThe authors declare they have no conflict of interest.