Hidradenitis suppurativa is a chronic inflammatory disorder that has attracted increasing attention in recent years due to underestimations of prevalence and the considerable impact of the condition on interpersonal relationships, physical appearance, self-esteem, and body image. Although hidradenitis suppurative has a significant psychological impact on patients and can even cause physical limitations when thick scarring results in limb mobility limitation, until very recently little evidence was available relating to its epidemiology, etiology, or pathogenesis. In this review, we highlight the latest advances in our understanding of the epidemiological and clinical aspects of hidradenitis suppurativa. We will also look at the different classification systems for hidradenitis suppurativa and discuss the emergence of skin ultrasound as a promising technique for monitoring the course of this chronic inflammatory disease.
La hidradenitis supurativa es un proceso inflamatorio crónico que en los últimos años ha adquirido una elevada importancia, por la subestimación de su prevalencia, y por tratarse de un proceso que produce una importante alteración en las relaciones interpersonales, de autoestima y de percepción de la imagen personal y de la imagen pública.
A pesar de ser un proceso patológico de elevada importancia, por su repercusión no solo psicológica, sino también por su posible limitación física, que puede llegar incluso a reducir de forma definitiva la movilidad en este tipo de pacientes debido a la formación de cicatrices retráctiles en áreas de movilidad de las extremidades, las evidencias con respecto a su epidemiología y a su etiopatogenia eran hasta hace bien poco escasas.
En la presente revisión se pretende analizar los últimos avances en el conocimiento de los diferentes aspectos epidemiológicos y clínicos de la hidradenitis supurativa. A su vez, se revisarán los diferentes sistemas de clasificación empleados actualmente en la evaluación de la gravedad de la enfermedad, así como la entrada de la ecografía cutánea como una técnica relevante en el seguimiento de este proceso inflamatorio crónico.
Hidradenitis suppurativa (HS) has been defined as a recurrent, debilitating chronic inflammatory skin disease that typically presents after puberty with deep, inflammatory, painful lesions in apocrine gland–bearing parts of the body; the most common areas affected are the axillae, the groin, and the anogenital region.1
HS has been historically referred to as Verneuil disease, after Verneuil, a French surgeon who linked the disorder to the apocrine glands in the mid 19th century and coined the term hidradenitis suppurativa.2 In later years, the pathogenesis of HS was attributed to follicular occlusion, and the condition was consequently included in the follicular occlusion triad, together with acne conglobata and dissecting cellulitis. This triad subsequently became a tetrad with the addition of a fourth condition, pilonidal sinus. In 1989, the term acne inversa was proposed as an alternative name,3 and ever since, with our growing knowledge of the causes and mechanisms involved, the denomination of the disorder has generated controversy.4 Some authors have even suggested that considering what we now know about the pathogenesis of HS, none of the terms currently in use are probably suitable.1
EpidemiologyMost epidemiological studies of HS have been conducted in Europe and the United States. The epidemiological data presented in this section are based on estimates for these populations, as we found no data for Spain in our review of the literature.
Prevalence figures for HS vary widely across the literature, probably because of differences in populations and methodologies. Numerous studies cite prevalence rates of between 1% and 4% based on the work of Jemec et al.,5 who calculated a 1-year prevalence of 1% and a point prevalence of 4.1% in the Danish population in the 1990s. In a later study, published in 2008, Revuz et al.6 estimated a prevalence of 0.97% based on the results of a survey of French individuals aged over 15 years. The only population-based study to investigate the prevalence of HS (conducted in the US state of Minnesota) reported an estimated prevalence of 0.13%, which was considerably lower than previous estimates.7 The difference can probably be explained by methodological differences (population-based study) and the fact that the other studies probably overestimated prevalence by basing their calculations on unconfirmed, self-reported data. Other US studies have reported rates of lower than 0.1%. Cosmatos et al.,8 for instance, reported a prevalence of 0.053% based on patient insurance claims data, while Shlyankevich et al.9 reported a rate of 0.08% in a retrospective case-control study.
Onset of HS typically occurs after puberty, typically at the beginning of the third decade of life, and the disease tends to remain active during the third and fourth decades of life. Many women with HS have been seen to experience an improvement on entering menopause and therefore patients aged over 50 with active disease are typically men.10
HS appears to be more common in women.11,12 According to the literature, the male to female ratio is approximately 3:1, with some of the more relevant studies reporting values in the range of 2.6:113,14 to 3.3:1.6
Very few studies have analyzed the distribution of HS by race or ethnicity, and objective data are scarce in this area. One recent study of the US population reported that HS was more common in black individuals.15
Epidemiologically, HS has been associated with multiple comorbidities,16–22 which are summarized in Table 1. While some of these conditions share pathological mechanisms or genetic factors with HS, in other cases the association is probably due to confounding factors.
Comorbidities in Hidradenitis Suppurativa.9–22
| Inflammatory bowel disease |
| Crohn disease |
| Ulcerative colitis |
| Endocrine and metabolic disorders |
| Metabolic syndrome |
| Cushing disease |
| Acromegaly |
| Thyroid diseases |
| Follicular occlusion syndromes |
| Acne conglobata |
| Dissecting cellulitis of the scalp |
| Pilonidal sinus |
| Genetic disorders associated with follicular occlusion |
| Pachyonychia congenita |
| Dowling-Degos disease |
| Steatocystoma multiplex |
| Joint diseases |
| Spondyloarthropathy |
| Psychiatric disorders |
| Depression |
| Anxiety |
| Alcohol or drug dependency |
| Neoplasms |
| Cutaneous carcinomas (squamous cell carcinoma) |
| Lymphomas |
| Dermatological diseases |
| Pyoderma gangrenosum (PASH syndrome) |
| Pityriasis rubra pilaris |
| Acanthosis nigricans |
| Panniculitis (erythema nodosum) |
| Fox-Fordyce disease |
| Kidney diseases |
| Nephrotic syndrome |
| Acute interstitial nephritis |
| Anemia |
| Amyloidosis |
| Polycystic ovarian syndrome |
| Behçet disease |
| Sjögren syndrome |
| PAPA syndrome |
| SAPHO syndrome |
| Down syndrome |
| Keratosis-ichthyosis-deafness syndrome |
Abbreviations: PAPA, Pyogenic arthritis, pyoderma gangrenosum, and acne; PASH, pyoderma gangrenosum, acne, and suppurative hidradenitis; SAPHO, synovitis, acne, pustulosis, hyperostosis, and osteitis.
HS is currently considered to be an inflammatory disease of the pilosebaceous follicle with an underlying immune system imbalance that occurs in genetically predisposed individuals. The course of disease is additionally modified by exogenous triggers or aggravating factors.23
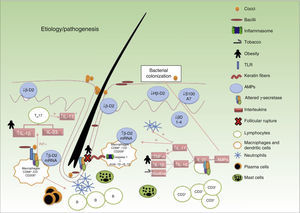
It is widely accepted that the sequence of events involved in the development of lesions is as follows (Fig. 1): 1) hyperkeratosis and follicular plugging; 2) dilation of the pilosebaceous unit; 3) follicular rupture and release of content into the dermis; 4) secondary inflammatory reaction; and 5) arrival of inflammatory cells and release of new cytokines, perpetuating the process. Nevertheless, the exact mechanism responsible for the chronic inflammation of the pilosebaceous unit that gives rise to the above cascade and perpetuates the process with the formation of abscesses and fistulous tracts is not fully understood.
The association between HS and autoimmune and autoinflammatory diseases, such as pyoderma gangrenosum and Crohn disease, together with clinical and laboratory findings, supports the existence of an immune system imbalance and consequently suggests inadequate control of the inflammatory response around the hair follicles in intertriginous areas.
Predisposing FactorsGenetic AspectsApproximately 40% of patients with HS have a family history of the disease.24 The most common pattern of inheritance is the autosomal dominant pattern, and the genes involved have been linked to the loci 1p21.1-1q25.3.24
Mutations that inactivate the presenilin1 gene (PSEN1), the presenilin enhancer gamma secretase subunit gene (PSENEN), and the nicastrin gene (NCSTN) have been described in families with severe, atypical clinical forms of HS. These genes code for 3 of the 4 subunits of ¿-secretase involved in the Notch signaling pathway. PSEN1, PSENEN, and NCSTN mutations have been associated with epidermal and follicular alterations, with absent or impaired formation of the sebaceous glands in mouse models.25–27
Interleukin 1βHS lesional and normal-appearing perilesional skin have been found to have significantly higher levels (31-fold) of interleukin 1β (IL-1β) than healthy skin, and IL-1β has also been found to be elevated in HS lesions compared with psoriatic lesions. In addition, IL-1β levels have been observed to correlate with symptom severity in HS,28 and to show greater reductions than other proinflammatory interleukins following treatment with tumor necrosis factor α (TNF-α) blockers.28 IL-1β is involved in most autoinflammatory processes (e.g. SAPHO, PAPA, PAPASH, and PASH syndromes), and is the therapeutic target of selective IL-1 receptor antagonists (anakinra).
TNF-αAlthough results vary across studies, elevated levels of TNF-α and TNF-α messenger RNA have been found in biopsy specimens of lesional and normal-appearing perilesional skin, and these levels are up to 5 times higher than those seen in psoriatic skin.29,30 Like IL-1β, TNF-α levels in HS lesional skin have also been seen to correlate with disease severity.31
Microbiome and BiofilmNormal human microbiome, or microbial flora, is formed by a series of symbiotic microbes that live inside humans, and alterations to this community have been linked to the development of autoimmune diseases such as inflammatory bowel disease.32,33
Several studies have demonstrated the presence of biofilms in the hair follicles and fistulous tracts of patients with HS.34–37 Although it is not known what role these biofilms play in the development of HS, logic would dictate that an imbalance in antimicrobial peptides would facilitate bacterial colonization, triggering an inflammatory cascade and the production of cytokines following pathogen recognition by macrophage toll-like receptors.37
Other Predisposing Factors (Table 2)38–46Triggers- a)
Tobacco. Approximately 70% to 88.9% of patients with HS are smokers.47–49 Nicotine stimulates IL-10 overproduction33 and is associated with impaired functioning of the γ-secretase and Notch signaling pathways.50
- b)
Obesity. Obesity is considered to be an aggravating rather than a triggering factor. As occurs with other autoimmune disorders, metabolic syndrome appears to be significantly associated with HS.51,52 It also influences mechanical irritation, occlusion, and maceration (Table 2).
Table 2.Other Predisposing Factors for Hidradenitis Suppurativa (HS).
Inflammation Pathway Alteration Reference Il -10 Overexpression of IL-10 in lesional and perilesional skin. IL-10 levels have also been associated with disease activity. Micheletti,36 Gold et al.37 IL-17 Increased expression (7-30 fold) of IL-17 and IL-17 mRNA in HS tissue compared with normal skin. Van der Zee et al.29 IL-12/23 Disparate results in the different tissue studies performed. Schlapbach et al.38 IL-22 High mRNA levels compared with healthy skin, but proportionally lower levels than in psoriasis or atopic dermatitis. Schlapbach et al.,38 Hofmann et al.39 β-defensin 2 High tissue levels of β-defensin 2 mRNA in areas with HS involvement. Emelianov et al.41 TLRs High TLR-2 levels (mRNA and protein) and suppression of other TLRs, particularly TLR-4. Schlapbach et al.38 Notch signaling pathway Altered suppressive action of Notch signaling pathway on TLR-4. Van Der Zee et al.,28 Van Der Zee et al.,29 Van Der Zee et al.30 Cellular immunity Unknown role of T lymphocytes in HS. Pink et al.26 Abbreviations: HS, hidradenitis suppurativa; IL, interleukin; mRNA, messenger RNA, TLR, toll-like receptor.
- c)
Hormonal factors. An association between HS and hyperandrogenism is supported by the predominance of HS in females, the occurrence of premenstrual flares, onset during menarche or adolescence, and improvements observed during pregnancy or after menopause in certain patients.47–49 However, treatment with oral contraceptives or 5α-reductase inhibitors has not achieved the expected results, and furthermore, the hyperandrogenism hypothesis is not supported by results from hormonal studies in patients with HS.47
- d)
Tight-fitting clothes. Shearing forces and friction can cause follicles to rupture, leading to the development of lesions.
- e)
Deodorants and depilation. Irritants such as deodorants and depilation products aggravate rather than trigger the disease.51,52
- f)
Drugs. Lithium, contraceptives, and isotretinoin are among the drugs that can trigger recurrent flares.51
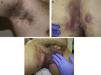
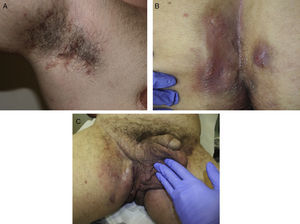
The clinical manifestations of HS are highly heterogeneous, but the disease tends to manifest with deep, painful, inflammatory lesions, including nodules, fistulas, and abscesses53 (Fig. 2 A-C). Double comedones are another characteristic finding and have been described as possible precursors of HS lesions in the skinfolds of children.54
Flares are associated with increased pain and oozing of pus and are common in women just before menstruation (40% of cases). They tend to spontaneously improve without treatment in about 7 to 10 days.55
HS generally appears in the second to third decade of life, although cases of early onset have been described in children.56 Early onset has been associated with greater involvement of lesions and increased genetic susceptibility, with a family history reported in 55% of patients with early-onset HS, compared with in 34% of patients with postpubertal HS.57,58
The most common sites of involvement are the axillae, the groin, the buttocks, the perianal and perineal areas, and the mammary and inframammary areas. Location of disease varies according to sex. While inframammary, axillary, and inguinal lesions are more common in women, lesions on the buttocks, in the perianal region, and at atypical sites (e.g. nuchal scalp and retroauricular areas) are more common in men.58–60 In 2013, Canoui-Poitrine et al.13 identified 3 phenotypes of HS, which they called LC1 (axillary-mammary class), LC2 (follicular class), and LC3 (gluteal class). LC1 patients typically have axillary and mammary lesions and a higher prevalence of hypertrophic scars,while LC2 patients typically have lesions on the ears, chest, back, and legs, in addition to follicular lesions (pilonidal sinus and comedones), severe acne, and a family history of HS. This phenotype was found to be more common in men and smokers, and was also linked to greater disease severity. Finally, LC3 patients tend to have gluteal lesions, papules, and folliculitis, as well as a lower prevalence of obesity and less severe disease.61 A new phenotype—HS fulminans—was proposed more recently, and reported to be more common in Afro-Caribbean men and to occur in association with rheumatological symptoms (arthritis and/or spondylitis) and anemia, in the absence of increased body mass index.62 It has been suggested that a phenotype-based classification could be of help for personalized therapy in HS.63
Histologic AspectsAlthough HS was initially considered to be a disease of the apocrine glands, it is currently defined as a follicular disease.64,65 Von Laffert et al.,66 on investigating the histologic findings for surgically excised HS lesions from 60 patients, reported follicular hyperkeratosis in 82% of cases, hyperplasia of follicular epithelium in 77%, and perifolliculitis in 68%. These 3 features would appear to correspond to the phase preceding rupture of the follicle structure. Other relevant features included a subepidermal interfollicular inflammatory infiltrate (78%) and epidermal psoriasiform hyperplasia with rete ridges of a similar length (58%). These last 2 structures were both present in 36% of the specimens studied.
In the same study, immunohistochemical staining of the follicular and subepidermal inflammatory infiltrate showed a very similar mixture of inflammatory cells, consisting of lymphocytes, neutrophils, plasma cells, and histiocytes. Another noteworthy finding is the presence of CD8+ lymphocytes with striking follicular and epidermal epitheliotropism.67 Finally, increased mast cells have been described in the dermis of early lesions and perilesional skin, possibly explaining, at least in part, why so many patients with HS report itching.68
A characteristic histologic finding associated with more developed lesions is the presence of fistulas with stratified squamous epithelium surrounded by fibrosis and inflammation.69 No differences have been found between patients with HS and controls in terms of the expression of estrogen or androgen receptors in the apocrine glands.70
Finally, it is important to note that squamous cell carcinomas may arise in HS lesions, typically in men with severe, long-standing disease with gluteal and perineal involvement. Although these carcinomas have a good histologic prognosis (well-differentiated or verrucous carcinomas), they are clinically aggressive (5-year survival, 61%) and are frequently associated with high-risk human papillomavirus infection.71
Disease Classification ModelsThere are various models for classifying and staging HS, including qualitative models, such as the Hurley Staging System, and quantitative models, such as the Sartorius and the modified Sartorius systems, the Hidradenitis Suppurativa Physician Global Assessment (HS-PGA), and the Hidradenitis Suppurativa Clinical Response (HiSCR) measure, among others. None of the systems are perfect, and each has its advantages and limitations. The most widely used scale in routine clinical practice is the Hurley Staging system, although some of the newer, more dynamic and practical systems, such as the HS-PGA and the HiSCR, are gaining ground.
The Hurley Staging SystemThe Hurley Staging system, proposed by Hurley in 1989, was the first classification model described for HS. It distinguishes between 3 levels of disease severity (Table 3).72–74 It is widely used because of its simplicity and speed, but it does have some limitations, including its qualitative, static nature. In other words, it does not take into account the number of body sites affected or the number of lesions at each site. It also contemplates certain fixed or invariable characteristics, such as scars and fistulas, and as such is not very useful for assessing treatment response.
Hurley Stages (Modified Table).
| Stage | Abscesses | Fistulous Tracts/Scarring | Prevalence2 |
| I | ≥1 | No | 7%-68% |
| II | Widely separated and recurrent | Minimal | 28%-83% |
| II | Multiple | Multiple | 4%-22% |
Years after publication of the Hurley Staging system, Sartorius developed a new, more detailed, system for assessing disease severity in HS. This system was subsequently modified by both himself (modified Sartorius score, Table 4)35,74,75 and Revuz (Sartorius score modified by Revuz).76
Sartorius Score Modified by Sartorius.
| Points | Points |
| Right Axilla | Left Axilla |
| Nodules and fistulas | Nodules and fistulas |
| Maximum distance | Maximum distance |
| Hurley III yes/no | Hurley III yes/no |
| Right groin | Left groin |
| Nodules and fistulas | Nodules and fistulas |
| Maximum distance | Maximum distance |
| Hurley III yes/no | Hurley III yes/no |
| Right buttock region | Left buttock region |
| Nodules and fistulas | Nodules and fistulas |
| Maximum distance | Maximum distance |
| Hurley III yes/no | Hurley III yes/no |
| Other locations | |
| Nodules and fistulas | |
| Maximum distance | |
| Hurley III yes/no | Total score |
| Parameters | Points per Parameter |
|---|---|
| 1. Number of areas affected | 3 |
| Three points per area | |
| 2. Number and severity of lesions | |
| Nodules | 1 |
| Fistulas | 6 |
| 3. Longest distance between 2 relevant lesions (or size if there is a single lesion) | |
| <5cm | 1 |
| 5-10cm | 3 |
| >10cm | 9 |
| 4. All lesions are clearly separated by normal skin | |
| Yes | 0 |
| No (Hurley III) | 9 |
| Patient-reported information (not included in the score): | |
| Number of boils in last month ____ | |
| Pain of most symptomatic lesion ____ | |
| Visual analog scale (0-10) | |
| The dermatologist makes a note of: |
| Affected regions: axillae, groin, buttocks (right/left), and other areas; 3 points per area |
| The number and type of lesions in each area, with the corresponding score (nodule, 1 point; fistula, 6 points) |
| The longest distance between 2 relevant lesions (or size if there is a single lesion) in each zone: <5cm, 1 point; 5-10cm, 3 points;>10cm, 9 points |
| Whether or not the lesions are separated by normal skin: if they are: 0 points; if they are not (Hurley III), 9 points |
| The total score is calculated by adding the points for each area |
| The pain or discomfort caused by the most symptomatic lesion at the time of the visit is assessed using a visual analog scale (0-10) |
In the original scoring system, each area affected by HS is evaluated separately, with different points assigned depending on the type of lesion present (abscess, draining fistula, nondraining fistula, inflammatory nodule, noninflammatory nodule, hypertrophic scar), the longest distance between 2 relevant lesions, and the presence of lesions separated by normal skin. An overall score is calculated by adding the total number of points.74,75
The modified Sartorius score is a simplified version that places more emphasis on the presence of inflammatory lesions in an attempt to make the system more useful for assessing treatment response (Table 4).76
Like the original system, the modified Sartorius score also takes into account the body areas affected, the number and type of lesions in each area, the distance between the 2 most relevant lesions, and the presence of normal skin separating these lesions. It also, however, takes into account the number of inflammatory lesions (nodules and fistulas) in 3 locations (axillae, groin, and buttocks). The system produces an overall score and a score per body area.76 Other recommendations include assessing pain using a VAS and considering the number of boils reported by the patient for the preceding month.76
Interobserver variability is low for the modified Sartorius score, and scores have been found to correlate positively with the presence of risk factors and other measures of severity, such as the Dermatology Life Quality Index.77 The use of this system, however, is limited in severe cases in which lesions that were originally separate eventually coalesce.77 While the modified Sartorius score is more dynamic than the Hurley Staging System, it also includes lesion characteristics that rarely vary in response to medical treatment (e.g., distance between 2 relevant lesions).74 A further limitation is that it does not assess the inframammary region as a separate region.
The modified system proposed by Revuz76 is similar, but assesses the perineal region, the inframammary and intermammary folds, and hypertrophic scars.
HS-PGAThe HS-PGA is one of the most widely used classification models to assess response to medical treatment in clinical trials.
It classifies disease severity into categories based on the number of abscesses, fistulas, inflammatory nodules, and noninflammatory nodules in all areas.78 The latest version of the HS-PGA classifies disease severity into 6 levels78 (Table 5).
Disease Severity Assessed Using the Hidradenitis Suppurativa Physician Graded Assessment (6 Categories).
| Category | Description |
| No lesions | 0 ABS, 0 DF, 0 IN, 0 NIN |
| Minimal | 0 ABS, 0 DF, 0 IN, ≥1 NIN |
| Mild | 0 ABS, 0 DF,<5 IN |
| 1 (ABS or DF), 0 IN | |
| Moderate | 0 ABS, 0 DF, ≥5 IN |
| 1 (ABS o DF), ≥1 IN | |
| 2–5 (ABS o DF),<10 IN | |
| Severe | 2–5 (ABS or DF), ≥10 IN |
| Very severe | >5 (ABS or DF) |
Abbreviations: ABS, abscesses; DF, draining fistula; IN, inflammatory nodules; NIN, noninflammatory nodules.
Source: Modified from Kimball et al.78
It is dynamic, simple, and quick to use, and is suitable for monitoring disease course. Its limitation is that it does not consider individual areas separately.
HiSCRRather than a classification model, the HiSCR is a measure for assessing response to medical treatment. It was recently validated and is designed to quantify disease severity and establish a meaningful clinical endpoint.79
The HiSCR is defined as a reduction of 50% of more in inflammatory lesion count (sum of abscesses and inflammatory nodules [AN]) and no increase in abscesses or draining fistulas when compared with baseline79 (Table 6).
The HiSCR therefore reflects a clinical endpoint based on total inflammatory lesion count in a patient with HS at a given moment. As a result, it allows for the calculation of percentage reductions in abscesses and inflammatory nodules with respect to baseline as follows: AN50 (50% reduction), AN75 (75% reduction), and AN100 (100% reduction).
The measure is particularly useful for assessing response to medical treatment, as it contemplates inflammatory lesions (not static lesions such as scars) and it is also quick to apply.
Hidradenitis Suppurativa Severity IndexThe Hidradenitis Suppurativa Severity Index includes objective and subjective categorical variables80 and has been used to assess the clinical efficacy of infliximab in 2 studies80,81 and of adalimumab in 1 study.82
The Role of Ultrasound in HSThe main limiting factor when assessing disease severity or disease activity is the presence of subclinical lesions that are not identifiable during the physical examination. Clinical palpation has poor sensitivity for differentiating between inflammatory nodules, noninflammatory nodules, and fistulas. However, this distinction is vital as the detection of fistulous tracts or fluid collections will necessitate changes to medical or surgical management.
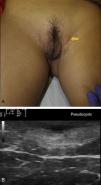
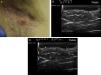
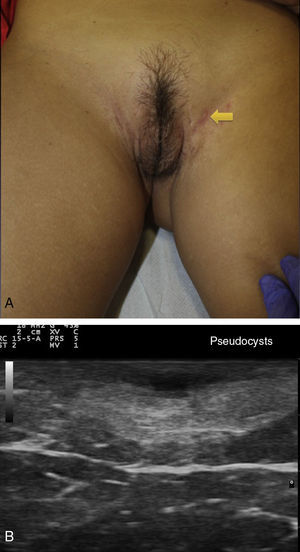
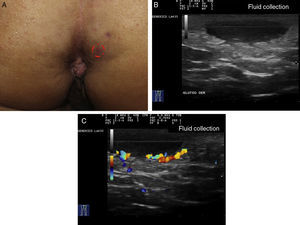
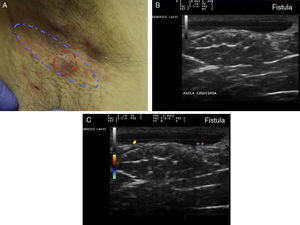
Ultrasound Criteria for Elementary HS LesionsThe main ultrasound findings in HS include diffuse alteration of dermal echogenicity patterns, dermal thickening, dermal pseudocysts, widening of hair follicles, and identification of fluid collections and fistulous tracts (Figs. 3–5).83
Hidradenitis suppurativa. A, Long erythematous lesion in the right armpit. The red circle shows the clinically evident area. The blue lines show the affected area as seen by ultrasound. B, Ultrasound image showing an underlying fistulous tract. C, Mild inflammatory activity evidenced by Doppler ultrasound.
The ultrasound findings corresponding to the different stages of clinical disease progression are described below. The earliest finding is hair follicle widening, which appears to have a key role in the development of HS.
The next stage consists of alteration of the dermal pattern (first perifollicular and then diffuse) and dermal thickening, which reflects the marked underlying inflammatory process, largely brought about by the action of varying innate immune system mediators. Accordingly, both degree of hypoechogenicity and the extent of the hypoechogenic area can provide important clues regarding the degree of underlying inflammation (Figs. 3–5).83
Patients with high inflammatory burden will have dermal pseudocysts (Fig. 3 A and B), which appear on ultrasound as round or oval hypoechoic or anechoic nodular structures. The next stage involves the development of fluid collections, which are seen on ultrasound as hypoechoic or anechoic fluid deposits in the dermis or hypodermis that are typically connected to the base of the altered hair follicle (Fig. 4 A-C).
The final stage is the development of fistulous tracts, seen as bands of hypoechoic or anechoic structures crossing through different structures located in the different layers of the dermis or hypodermis, and connected to the base of altered follicular structures (Fig. 5 A-C).
Proposed Clinical-Sonographic Scoring System for HSUsing the ultrasound findings for HS lesions in 34 patients, Wortsman et al.83 developed a clinical-sonographic scoring system (SOS-HS) for staging HS (Table 7). Although the system has not yet been validated, it may be useful for follow-up and monitoring in this setting.
Ultrasound Staging of Hidradenitis Suppurativa (Clinical-Sonographic Scoring System in HS).
| Stage | Description |
| I | Single fluid collection and dermal changes (hypoechoic or anechoic pseudocysts, widening of hair follicles, altered dermal thickness)Involvement of a single body area, i.e. axilla, groin, breast, buttock (unilateral or bilateral)No fistulous tracts |
| II | Between 2 and 4 fluid collections or a fistulous tract, with dermal changesInvolvement of 1 or 2 body areas (unilateral or bilateral) |
| III | ≥5 fluid collections or ≥2 fistulous tracts, with dermal changesOr involvement of ≥3 body areas (unilateral or bilateral) |
Source: Modified from Wortsman.80
HS is a relatively common chronic inflammatory disease with clearly defined clinical manifestation patterns. Familiarity with these patterns should help to reduce the diagnostic delays that are sometimes seen in HS and can cause considerable limitations for patients. Cutaneous ultrasound can help to assess the true burden of disease, as HS is characterized by deep-seated lesions that are often not clinically identifiable.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Martorell A, García-Martínez FJ, Jiménez-Gallo D, Pascual JC, Pereyra-Rodriguez J, Salgado L, et al. Actualización en hidradenitis supurativa (I): epidemiología, aspectos clínicos y definición de severidad de la enfermedad. Actas Dermosifiliogr. 2015;106:703–715.