The human microbiome includes viruses, bacteria, and fungi. There is evidence that in addition to microbiome variation in different areas of the body or according to ethnicity and sex, the microbiome specific to the scalp is conditioned by such factors as humidity, protection from UV light, and pH. Although little information has yet been published about the microbiome of hair follicles and its role in the pathogenesis of diseases, interest in this area of research is emerging. Studies have shown that components of the follicular microbiome influence such disorders as androgenetic alopecia and alopecia areata. A current hypothesis is that interventions that target the microbiome may lead to innovative therapies for many diseases.
El microbioma incluye microorganismos como virus, bacterias y hongos. Se ha evidenciado que el cuero cabelludo tiene su propio microbioma dado por factores únicos como humedad, protección de luz ultravioleta y pH, adicionalmente existen diferencias entre distintas áreas corporales, etnias y sexos. Existen pocas publicaciones o datos sobre el microbioma folicular y se ha denotado el rol de la microbiota en la patogénesis de varias enfermedades siendo un área de investigación emergente. Algunos estudios demuestran la influencia de esta composición con enfermedades capilares como la alopecia areata y alopecia androgenética. Finalmente se ha postulado que la manipulación del microbioma puede representar una opción terapéutica innovadora para muchas enfermedades.
The microbiome describes the genome of all commensal, symbiotic, and pathogenic organisms in the human body, including viruses, bacteria, and fungi. The composition is influenced by such factors as age, nutrition, gender, and lifestyle. The rise in the prevalence of autoimmune diseases, especially in Western countries, has been thought to be related to modern lifestyles, their effect on the normal flora of the body, and consequent immune dysregulation. A state of dysbiosis can trigger T-cell dysregulation and cause a variety of disorders locally or at a distance. These changes in the intestinal or cutaneous microbiota can alter cell differentiation in both the innate and adaptive immune systems. Microbiota also modulate epithelial secretion of chemokines that attract immune cells.1 Scientists who are currently exploring how the gut microbiome can influence immunity at remote sites such as the skin have proposed the existence of a “skin-gut axis.”2
An infectious cause of alopecia areata (AA) was hypothesized by David Gruby after he demonstrated the presence of Microsporum audouini around the hair follicles of individuals with the condition; the species was then blamed for epidemics in schools and orphanages.3 However, while some dermatologists did isolate diverse fungi in patients with AA, others failed to find associations between the disease and pathogens; as a result, attention was drawn away from a role for infection.3 Nevertheless, there could be a possible role for pathogenic antigens and their increased activity in the immune system, which could induce AA via epitope similarity.3 Another suggested role for gut microbiota is an effect on the differentiation of peripheral lymphocytes, given that some regulatory T cells (Tregs) could be redirected to a part of the intestine where molecular and microbial signals convert forkhead box P3 negative (Foxp3–) CD4+ Treg cells to Foxp3+ ones.4
The role of microbiota in the pathogenesis of various diseases is an emerging area of research.1 Doubts remain about whether dysbiosis is a primary cause of disease or a secondary one associated with changes caused by a primary mechanism,1,5 but the possibility that therapeutic manipulation of the microbiome might be an option in conditions such as AA has been suggested.5 This review summarizes recent evidence on the associations between the microbiome, the process of dysbiosis, its relation to alopecia, and the implications for therapy. We also discuss the composition of the microbiome and differences between that of healthy persons and patients with various types of alopecia.
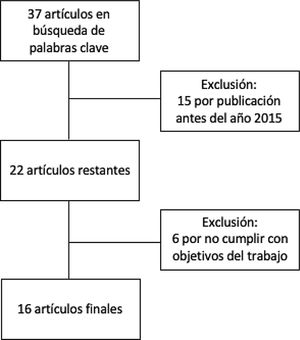
MethodsThis is a narrative review of the subject. We obtained articles published between 2015 and 2020 by searching in the Cochrane Library Plus, Scielo, DynaMed, PubMed, and Google Scholar. The search terms were microb* AND alopecia. To identify the articles relevant to the purposes of the review (Fig. 1), we read abstracts and results, and when necessary, the full texts to ascertain which ones contained pertinent information.
ResultsNormal Microbiome of the Skin and Hair FolliclesThe scalp has its own microbiome6 in a humid environment protected from UV light and with a pH favorable to microbial growth. The environment contrasts with that of other skin surfaces, which are dry, exposed to UV light, and have a low pH. There exist additional differences related to area of the body, ethnicity, gender, and the environment.1
Few studies have produced data on the microbiome of hair follicles. However, there is evidence that bacteria of the phyla Actinobacteria, Firmicutes, and Proteobacteria predominate on the surface of the scalp1 and that the most abundant organisms are Cutibacterium species (especially Cutibacterium acnes) and Staphylococcus species (especially Staphylococcus epidermidis). Other bacteria found on the scalp are Corynebacterium, Streptococcus, Acinetobacter, and Prevotella species.7,8 The most prevalent fungi on the scalp are Malassezia globosa and Malassezia restricta. Fungi in 2 phyla are present: Ascomycota (Acremonium species, Didymella bryoniae, Coniochaeta species, and Rhodotorula species) and Basidiomycota (Cryptococcus liquefaciens and Cryptococcus diffluens).7,8
Scalp colonization by eukaryotic DNA viruses such as adeno-associated virus subtype 21 and the human papilloma virus has been reported.1,7Demodex folliculorum has been found in the follicular infundibulum and Demodex brevis in the sebaceous glands. In addition, Naspitz et al7 found Dermatophagoides and Euroglyphus species on the scalp. Although it is not known whether these microorganisms contribute to the physiology of the hair follicle, it is known that they can generate proinflammatory and immunosuppressive responses.1
Dysbiosis and DiseaseAlopecia AreataOne study that sequenced the scalp microbiome of healthy subjects and patients with AA found between-group variation in bacterial populations: the proportions of bacteria in the Actinobacteria and Firmicutes phyla, respectively, were 57.4% and 29.2% in the AA group and 56.3% and 35.2% in the control group.6 The proportions assigned to Propionibacterium and Staphylococcus species in the AA group were 55.1% and 27.4%, respectively, and 45.6% and 32.6% in controls.6 (Some patients with AA are known to experience a significant increase in P acnes.1)* Alpha diversity was also higher in the patients, in whom there was a decrease in the proportion of S epidermidis,6 while Staphylococcus aureus proportions remained stable.6 The authors found no significant differences in the S epidermidis/S aureus ratio, whereas the P acnes/S epidermidis and P acnes/S aureus ratios were higher.6 Polak-Witka et al7 have noted that it is possible that S aureus superantigens, proteases, and toxins, along with direct interactions between immune cells and bacteria, may contribute to inflammation, altering the skin’s barrier function and affecting the follicular region.
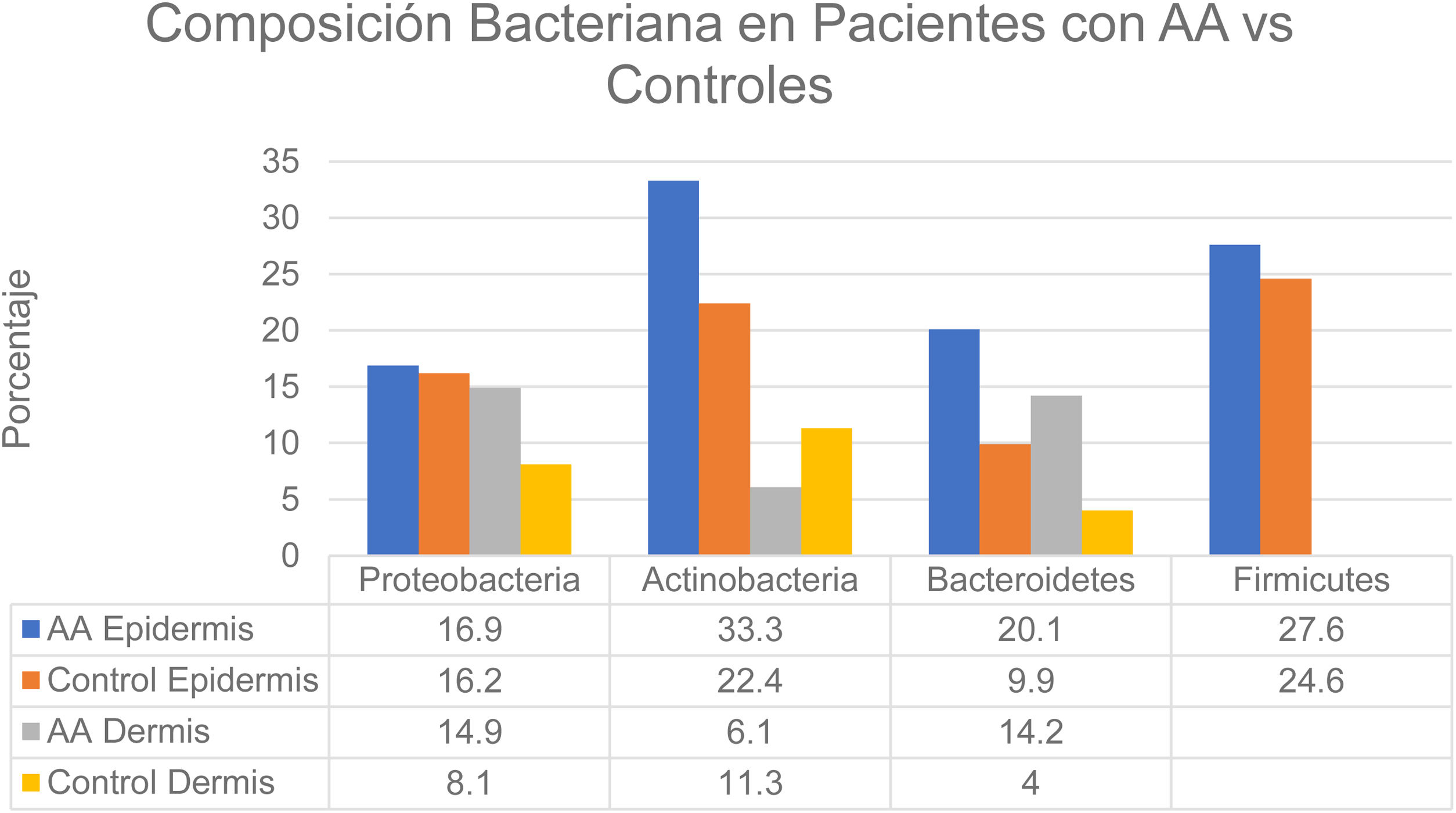
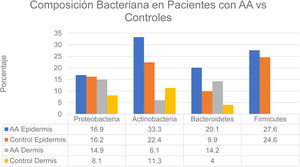
Changes in the microbiome of different compartments of the skin were also observed in the study.6 The proportions of bacteria on the epidermis assigned to the Actinobacteria and Bacteroidetes phyla, respectively, were 33.3% and 20.1% in the AA group and 22.4% and 9.9% in controls. In the dermis the proportions were as follows: Actinobacteria, 6.1% in AA and 11.3% in controls; Proteobacteria, 14.9% in AA and 8.1% in controls; and Bacteroidetes, 14.2% in AA and 4.0% in controls (Fig. 2). The bacterial proportions in the hypodermis were higher in patients with AA than in controls for the following phyla: Proteobacteria, Bacteroidetes, and Firmicutes. Finally, Prevotella copri was detected in all compartments analyzed in patients with AA, and Akkermansia muciniphila (<1.5% of the total microbiome) was detected in subcompartments (especially the hypodermis).
Distribution of bacterial colonization of the scalps of patients with AA and controls. AA refers to alopecia areata. Data source: Pinto et al.6
The association between AA and Helicobacter pylori is disputed. Evidence of a role for this bacterium in various autoimmune conditions has been reported, and the resolution of alopecia after treatment for the infection has been observed, although the exact mechanism responsible is unknown.9
AA has been linked to certain viral infections, such as swine flu (during the 2009–2010 outbreaks), other influenza infections, and mononucleosis (infection by the Epstein-Barr virus); a role for cytomegalovirus infection has also been debated.7,9 Cytomegaloviral DNA has been found in the follicular tissues of patients with AA.7 Finally, a possible connection between this form of alopecia and infection by Alternaria species is possible, based on positive cultures in 20% of cases versus 13.3% of controls.7
In addition to the cutaneous microbiome, the gut microbiome may be involved in AA. Increased permeability of the intestine due to dysbiosis and/or inflammation has been demonstrated, and it may place stress on the immune system in genetically susceptible individuals. Dysbiosis leads to a reduced production of short-chain fatty acids as a result of inadequate intake of fiber in so-called Western diets, altering the intestinal barrier and affecting Tregs, which modulate the immune system.7 Patients in this state have been found to have increased populations of Holdemania filiformis, bacteria in the Erysipelotrichacea and Lachnospiraceae families, Parabacteroides johnsonii, Clostridiales vadin BB60 group, Bacteroides eggerthii, and Parabacteroides distasonis.10 The same study also found an association between these colonizations and the presence of AA. Finally, the presence of Lactobacillus species in the gut has been shown to be essential to the induction of this type of hair loss.11
Androgenetic AlopeciaEvidence points to the existence of microinflammation when multiple organisms are present in the superior third of the hair follicle, where Cutibacterium species have been found in 58% of patients with androgenetic alopecia (AGA) versus 12% of controls, according to literature reviewed by Polak-Witka et al.7 These species secrete porphyrins, which stimulate complement activation. Moreover, symptom improvement has been observed after application of antimicrobial agents, supporting the likelihood of a role for scalp microbiota.
A high load of P acnes in the follicles of miniaturized hairs of patients with AGA has been hypothesized.1 Species in the phyla Actinobacteria, Firmicutes, and Proteobacteria were shown to account for 98% of the scalp microbiota both on healthy scalps and in AGA in one study.12 The authors reported that species in the genera Propionibacterium and Staphylococcus account for about 90% of the bacterial load in both healthy subjects and individuals with AGA. The distributions of species in these 2 genera were similar in both groups, at 79% and 12%, respectively, for healthy subjects and 76.5% and 14%, respectively, in AGA. Individuals with AGA had increased presence of Stenotrophomonas geniculate, and the C acnes/S epidermidis ratio was also higher than in control subjects.
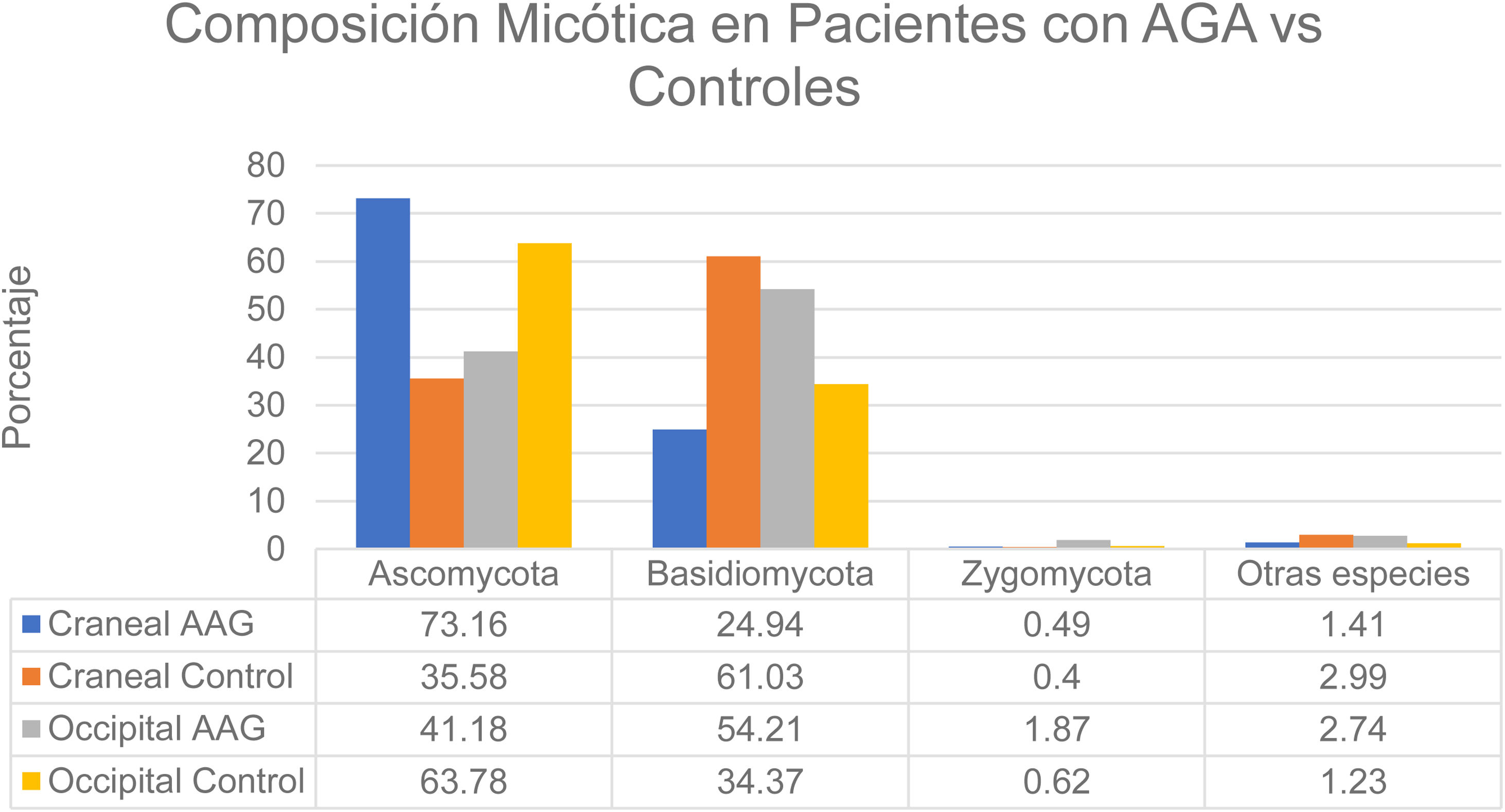
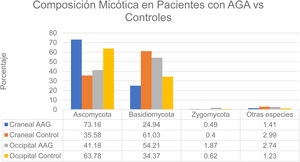
That study enrolled men with grade 3-4 alopecia on the Hamilton-Norwood scale and women with grade II hair loss on the Ludwig scale.12 The patients refrained from treating their hair with permanents or dyes for 2 months before the study started. They also refrained from using hair-loss shampoos and oral or topical antifungal treatments for 1 month. None had a history of scalp diseases such as folliculitis, lice infestation, or AA. The authors formed 2 groups: patients with alopecia and controls. A higher rate of bacterial colonization was found in the AGA group (60%) than in the controls (40%). The species found on the vertex of the scalp in controls belonged to the phyla Ascomycota (73.16%), Basidiomycota (24.94%), and Zygomycota† (1.41%). The occipital scalp region in the control group was colonized by microbes in these phyla in the following proportions: Ascomycota, 63.78%; Basidiomycota, 34.37%; Zygomycota, 0.62%; and other, 1.23%. At the crown of the scalp, species in these phyla accounted for the following proportions of fungal colonization in patients with AGA: Ascomycota, 35.58%; Basidiomycota, 61.03%; Zygomycota, 0.40%; and other, 2.99%. Occipital colonization by species in these phyla in AGA patients was distributed as follows: Ascomycota, 41.18%; Basidiomycota, 54.21%; Zygomycota, 1.87%; and other, 2.74% (Fig. 3). The Malassezia species load at the vertex was significantly greater in the men with AGA than in the control group, but the loads were similar in the occipital region in both groups. The load was significantly higher at the vertex than in the occipital region in the patient group; in contrast, the 2 scalp regions had similar fungal loads in the control group.12 Although M restricta and M globosa have been reported to be among the most abundant species on the scalp,1 some researchers have observed lower proportions of M globosa and M restricta in AGA patients (52%) than in controls (56%).8 It has been suggested that Demodex species play a role in AGA and seborrheic dermatitis.1
Distribution of fungal colonization of the scalps of patients with AGA and controls. AGA refers to androgenetic alopecia. Data source: Huang et al.12
Whether the massive bacterial superinfection that is associated with some scarring alopecias is part of a primary pathogenic mechanism or is secondary to inflammatory changes that lead to hair follicle dysbiosis is a subject of debate. This is the case for folliculitis decalvans (FD). The fact that antibiotic therapy by itself is usually ineffective and the condition responds better to retinoids does not rule out dysbiosis as the possible trigger for neutrophilic folliculitis, involving, for example, keratinocytes that secrete neutrophil-attracting cytokines and chemokines.1 The presence of S aureus in cultures and a temporary response to antibiotic treatment points to infection as the trigger.7
A Spanish study in patients with the typical neutrophilic pattern of FD demonstrated the presence of Staphylococcus species in 25.9% of diseased hair follicles biopsied from affected parts of the scalp.12 These bacteria were present in only 6.6% of the healthy follicles. However, S aureus infection is opportunistic in FD rather than causative, given that some patients show active disease even in the absence of significant levels of this pathogen, especially in lichenoid forms of FD. In addition, these bacteria have been shown to be no more virulent than others in the general population. Therefore, a hypothesis that emerges is that immunologic vulnerability or an altered hair follicle structure leave an individual susceptible to scarring alopecia. The findings of this study contradict earlier theories about how FD develops.
Moreover, Yip et al14 found that 80% of patients were colonized by S aureus on both lesional and healthy skin. They also demonstrated subepidermal colonization by S aureus. Invasion by this opportunistic species, which is the only one that has been shown to colonize over two thirds of patients with FD, points to a compromised epidermal barrier, as mentioned previously. The same study showed that antibiotic treatment did not fully restore the microbiota in patients with FD, suggesting a persistent defect in the epidermal barrier in this disease.
Finally, C acnes bacteria have been observed to form biofilm-like structures in biopsy samples.7,14 Such biofilms may not initially appear to be pathogenic, but in addition to causing inflammation they provide a stable, protective environment from which bacteria can spread out and cause disease.7,14,15 Antibiotic treatment can eliminate bacteria released from biofilms and alleviate symptoms, but those that remain inside may still constitute a source of chronic infection.7
FD was classified as a predominantly neutrophilic scarring alopecia in the past, but has recently been described as having a wide spectrum of presentations, including the possible co-occurrence of lichen planopilaris (LPP).13 Trichoscopy has revealed a dynamic shift in the FD phenotype toward LPP over time in this variant of liquenoid alopecia.13,15 One group proposed the Koebner phenomenon or the presence of an unrecognized secondary infection of LPP as mechanisms for chronic inflammation in FD.15 They also suggested the possibility of a phenotype of FD that includes LPP, in which both have a common origin in microbial dysbiosis that causes follicular damage or stress, leading to an inflammatory response and potential exposure to autoantigens and abnormal immune responses.15
Matard et al13 showed that patients with the FD-LPP had lower loads of S aureus (accounting for less than 20% of the follicular microbiota) than patients with classic FD, in whom the proportion exceeded 20%. Patients with the FD-LPP variant responded to oxacillin and lack the methicillin resistance (mecA) and Panton-Valentine leukocidin (PVL) genes. The authors also suggested that classical FD, characterized by a predominantly neutrophilic infiltrate, is best distinguished from the FD-LPP clinical variant by the presence of staphylococci. This difference suggests that the more acute neutrophilic form could be managed with antibiotics in its acute phases, whereas antiinflammatory agents might be the first line of therapy for managing the FD-LPP variant.
Advances in TherapyFecal TransplantationThree case reports have described men with AA who experienced sustained follicular growth in response to fecal transplantation, supporting the theory that gut microbiota play a role in inducing this type of hair loss.7,16,17 Two of the men had the universalis form of AA.7,16 In the first case, a 38-year-old man whose condition was refractory to intralesional corticosteroid therapy, reported hair growth on his arms and head (including his face), which was confirmed at a clinical appointment 8 weeks later.16 The response was maintained 3 years later. The second was a 20-year-old man with severe ileocolitis (Crohn disease) as well as a 2-year history of AA resistant to therapy with intralesional and topical corticosteroids, stearic acid, and laser.16 He had received treatment for recurrent diarrhea due to Clostridium difficile. After a fecal transplant, his severity of alopecia (SALT) score improved from S4b (95% to 99% hair loss) to S2 (25% to 49% loss). The patient in the third case was an 86-year-old man with a history of noninfectious diarrhea associated with intestinal dysbiosis who also reported hair growth on his scalp and a change in color from white to black in some residual hairs without direct treatment of the scalp.17 The response to treatment was long-lasting in this case too.
Use of PostbioticsPlatelet-rich plasma therapy is used in various types of alopecia. However, the treatment has limitations, among which is the variability of formulations (lack of a standardized platelet concentration). Modern biotechnology has created bioactive peptides able to simulate the activity of platelet growth factors. These peptides have similar efficacy and can be used topically; formulations for intralesional application may come in the future.18
Theories of an association between altered gut microbiota and hair loss, particularly in AA, have suggested a possible therapeutic role for microbial metabolites known as postbiotics. A double-blind placebo-controlled trial enrolling 160 participants with AA (SALT scores, S2–S5) aged 18 to 60 years assigned the treatment group to receive a preparation containing plantaracin A, Lactobacillus kunkeei, and an extract of Tropaeolum majus flowers.18 The 2 groups had similar demographic characteristics and histories of disease refractory to topical and systemic treatments as well as phototherapy. They had not received treatment in the year prior to enrollment in the trial. Complete resolution of symptoms was observed in 47.50% in the treatment group and partial resolution in 13.75%; 6.25% of the actively treated participants had no response. In contrast, only 5% of the control participants achieved complete resolution.
A large number of biomimetic peptides have been developed in an effort to overcome the limitations of platelet-rich plasma. Short chains of 10 to 15 aminoacids have been reported to behave similarly to natural growth factors by simulating their structure, their activity, or both.18 These peptides have greater stability and specificity than platelet-rich plasma, are more economical, and can be more easily incorporated into topical formulations.
Another study investigated the effect of probiotics on generating short-chain fatty acids, butyrate in particular.11 Chronic AA was not reversed in 16 weeks of treatment, but the Tregs/CD4+ ratio in cutaneous lymph nodes did improve in 15% of those in the treatment group (vs in 12% of controls).
Other Possible Future TreatmentsDiviccaro et al19 studied the long-term effect on microbiota of discontinuing finasteride in a rat model evaluating stool samples 1 month after stopping treatment. The alpha diversity did not change, but bacteria in the phylum Firmicutes decreased from baseline, whereas those in the phylum Bacteroidetes increased. Bacteria in the Bacteroidaceae and Prevotellaceae families were the most abundant, followed by species in the Lactobacillaceae, Lachnospiraceae, and Ruminococcacea families. Bacteroides, Prevotella, Lactobacillus, Oscillospira, Lachnospira, Ruminococcus, and Coprococcus species were most prevalent. In contrast, Oscillospira and Lachnospira species significantly declined in the absence of finasteride. Finally, in the group still on finasteride, bacteria in the Bacteroidetes phylum and the Prevotellaceae family increased, supporting the hypothesis that exposure to finasteride can affect the composition of gut microbiota.
Borde and Åstrand11 noted in a review of novel therapies that mammalian G-protein-coupled receptors 41 and 43 are possible therapeutic targets in immune disorders. Although their role is still not clear, the authors note, they cite GPR43-deficient mouse models that have demonstrated exacerbated or persistent inflammation in colitis, arthritis, and asthma, suggesting that this receptor may aggravate inflammatory diseases. Moreover, intestinal propionate may stimulate GPR43/41 or GPR43/109), inducing more Tregs that can protect hair follicles from an immune attack. The reviewers describe a pilot study that saw hair regrowth in 5 out of 5 mice 11 weeks after treatment with propionate versus none in the control group. After 4 more weeks, the researchers’ analysis of differences in cellularity with treatment found a large increase in the Treg/CD4+ ratio versus the control group. An attempt to replicate that study, however, could not produce the same effects on hair growth. Finally, prophylactic treatment was unable to prevent the development of disease symptoms.
Polyphenols and terpenes have been said to have a positive effect on cells involved in follicular growth, by intensifying cellular proliferation in dermal papillae cells and increasing the concentrations of factors such as type 1 insulin-like growth factor and vascular endothelial growth factor, reducing oxidative stress and improving hair growth.8
One study enrolled 12 patients with AGA between 40 and 65 years of age and classified as having stage 3 or 4 hair loss on the Hamilton-Norwood scale.8 When entering the study, the patients had not used antibiotics in the previous 30 days, had not used probiotics within 15 days, and had not shampooed within 48 hours. They had not undergone antitumor, immunosuppressant, or radiotherapy (3 months), used topical or hormone treatments for hair growth (3 months), or used minoxidil or finasteride (6 months). Nor did they have a history of other progressive dermatologic or inflammatory diseases affecting the scalp (such as psoriasis; seborrheic dermatitis; or severe erythema, excoriation, or sunburn). A phototrichogram was used to assess hair density on days 1 and 84, focusing mainly on Hamilton-Norwood stage 3 hair loss. A preparation containing a 1% extract of Lindera strychnifolia and a placebo preparation were applied twice daily for 84 days on 2 areas of the scalp (treatment and placebo areas). Application of the active treatment did not affect alpha diversity but did achieve maintenance of bacterial biodiversity. The researchers reported a significant decrease in C acnes (15%), an increase in S epidermidis (33%), and a decrease in the C acnes/S epidermidis ratio (37.8%). The L strychnifolia application reestablished “normal” fungal populations, especially fungi of the Basidiomycota phylum and 3 genera (Wallemia, Eurotium, and Malassezia). The researchers observed abundant Malassezia species, which increased by 3%, and M restricta loads were restored to the level of the healthy controls. Eurotium and Wallemia species were seen to decrease. Finally, hair growth increased significantly, by 7%.
DiscussionThe skin microbiome is a complex microbiological system with multiple interactions that can lead to both local and distant changes in homeostasis. Imbalances can trigger disease processes such as immune cell dysfunction and can also lead to functional changes, such as in hair follicles. Therefore, the system may be associated with inflammatory processes, such as AA, or changes in the hair follicle cycle, as in AGA. Multiple changes in skin surface or scalp microbiota, whether bacterial or fungal, have been described in both AA and AGA. However, the meaning of the associations remains uncertain, and the issue is complicated by our poor understanding of the follicular microbiome. The microbiology of the hair follicle must be better profiled so that we can learn more about its possible role in pathogenesis. The field of research is still fairly wide, and the possible therapeutic applications are many.
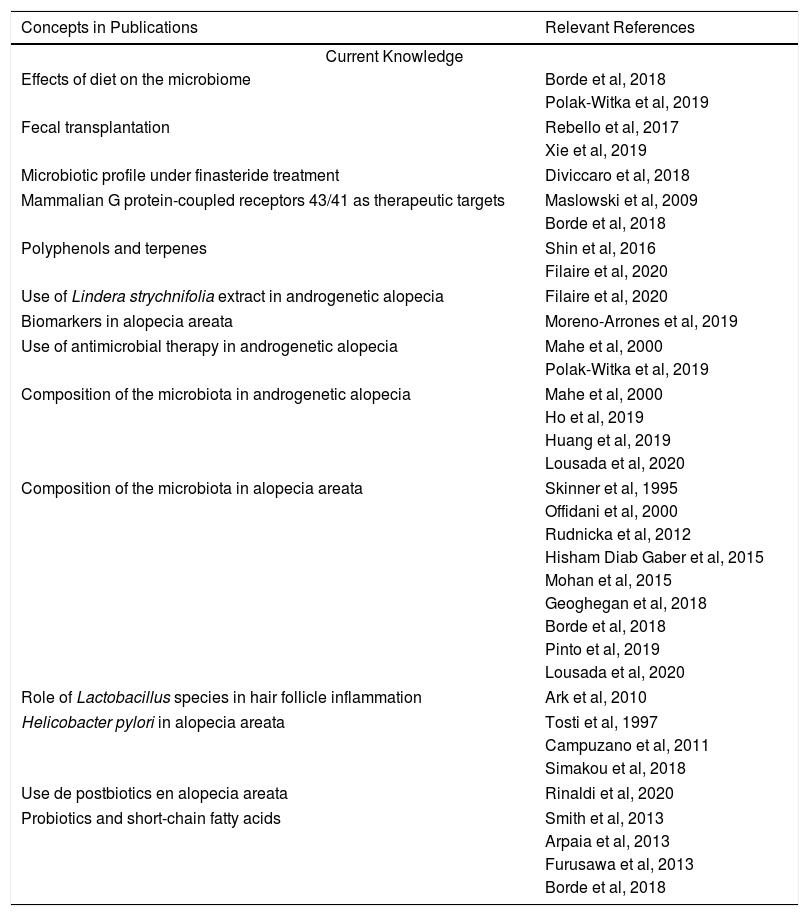
In the profiles described above, the importance of changes in both the genera and species of bacterial populations associated with AA must be emphasized. Also important are cases in which disease has regressed after the use of postbiotics or bacterial therapies in association with conventional therapies such as platelet-rich plasma. Other common therapies can also be considered: corticosteroids can change the proportional distribution of microbes and also play an immunomodulatory role, leading to questions about the mechanism by which they act as either modulators or triggers of disease. Cases in which improvement has followed fecal transplantation have been reported, introducing another possible therapeutic target: gut microbiota. However, so far, studies have involved few patients and must be replicated in larger trials before efficacy and possible adverse effects can be evaluated. Finally, the literature in this novel area focusing on the role of the microbiome is scarce in certain diseases. Currently, more is known about the microbiome’s role in inflammatory diseases such as atopic dermatitis and psoriasis. We therefore emphasize that this line of of research holds promise for finding novel therapeutic targets in the gut or cutaneous microbiomes. Possibilities include local, systemic, and diet therapies as well as the use of live organisms such as those found in probiotics (Table 1).
Current Knowledge and Lines of Research for the Future
| Concepts in Publications | Relevant References |
|---|---|
| Current Knowledge | |
| Effects of diet on the microbiome | Borde et al, 2018 |
| Polak-Witka et al, 2019 | |
| Fecal transplantation | Rebello et al, 2017 |
| Xie et al, 2019 | |
| Microbiotic profile under finasteride treatment | Diviccaro et al, 2018 |
| Mammalian G protein-coupled receptors 43/41 as therapeutic targets | Maslowski et al, 2009 |
| Borde et al, 2018 | |
| Polyphenols and terpenes | Shin et al, 2016 |
| Filaire et al, 2020 | |
| Use of Lindera strychnifolia extract in androgenetic alopecia | Filaire et al, 2020 |
| Biomarkers in alopecia areata | Moreno-Arrones et al, 2019 |
| Use of antimicrobial therapy in androgenetic alopecia | Mahe et al, 2000 |
| Polak-Witka et al, 2019 | |
| Composition of the microbiota in androgenetic alopecia | Mahe et al, 2000 |
| Ho et al, 2019 | |
| Huang et al, 2019 | |
| Lousada et al, 2020 | |
| Composition of the microbiota in alopecia areata | Skinner et al, 1995 |
| Offidani et al, 2000 | |
| Rudnicka et al, 2012 | |
| Hisham Diab Gaber et al, 2015 | |
| Mohan et al, 2015 | |
| Geoghegan et al, 2018 | |
| Borde et al, 2018 | |
| Pinto et al, 2019 | |
| Lousada et al, 2020 | |
| Role of Lactobacillus species in hair follicle inflammation | Ark et al, 2010 |
| Helicobacter pylori in alopecia areata | Tosti et al, 1997 |
| Campuzano et al, 2011 | |
| Simakou et al, 2018 | |
| Use de postbiotics en alopecia areata | Rinaldi et al, 2020 |
| Probiotics and short-chain fatty acids | Smith et al, 2013 |
| Arpaia et al, 2013 | |
| Furusawa et al, 2013 | |
| Borde et al, 2018 | |
| Lines of Future Research |
|---|
| Randomized controlled trials of recently discovered treatments |
| Role of diet modifications: their effects on the composition of microbiota and their therapeutic uses |
| Better descriptions of changes in the microbiome and their pathogenic roles |
| Research on possible therapeutic uses of probiotics and postbiotics |
| Adverse effects of experimental treatments |
| Research on the effects of conventional treatments and how they change the microbiota |
| Uses of biomarkers and precision medicine |
| Molecular and cellular therapeutic targets |
| Role of the gut microbiome and its implications |
| Use of fecal transplantation as an alternative therapy |
| Cost-effectiveness comparisons between new therapies and current ones |
The authors declare that they have no conflicts of interest.
Translator’s note: Cutibacterium acnes is the name by which Propionibacterium acnes is known in the current nomenclature. The authors use both names in the article. For the sake of preserving consistency between this translation and the original Spanish version, the translation follows the authors’ usage.
Please cite this article as: Barquero-Orias D, Muñoz Moreno-Arrones O, Vañó-Galván S. Alopecia y microbioma: ¿futura diana terapéutica? Actas Dermosifiliogr. 2021;112:495–502.
Translator’s note: The former phylum Zygomycota is currently divided into 2 phyla (Mucoromycota and Zoopagomycota). This translation follows the authors’ use of nomenclature.