Lichenoid eruptions (LE) pose a real diagnostic challenge due to their clinical and histopathological similarity to lichen planus. Inducers are varied being drug-induced lichenoid eruption (DILE) a common entity, particularly in an era characterized by the emergence of biologic or targeted therapies. This is the case of an alirocumab-induced lichenoid eruption, a proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitor used to treat dyslipidemia, a drug rarely associated with local skin adverse reactions and exceptionally with urticaria, or vasculitis.
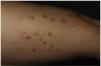
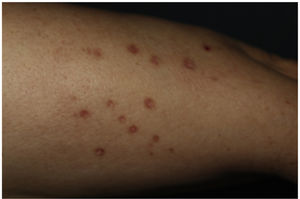
This is the case of a 61-year-old woman with a past medical history of mixed dyslipidemia and statin intolerance on an 18-month course of subcutaneous alirocumab, who presented with a 2-month history of highly pruritic eruption on her extremities. Examination revealed the presence of erythematous-violet, shiny, polygonal papules with Wickham striae on both her legs and forearms (figure 1). No lesions on mucous membranes were reported.
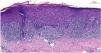
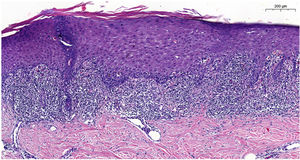
Blood tests results showed triglyceride levels at 358mg/dL (< 150) and LDL at 215mg/dL (< 130). The rest of the study, including a complete blood count, biochemistry, and autoantibodies was normal and tested negative for hepatotropic viruses. Dermatopathological examination revealed the presence of lichenoid interface dermatitis, a few necrotic keratinocytes, and vacuolar degeneration of the basal layer (figure 2). All these findings led to the diagnosis of alirocumab-related DILE.
A 15-day systemic course of prednisone plus with topical betamethasone was started—without alirocumab discontinuation—which achieved complete remission at 3 weeks that was maintained at the 14-month follow-up.
Alirocumab is a monoclonal IgG1 antibody vs PCSK-9 that inhibits its binding to the LDL receptor, thus increasing the number of available receptors for LDL removal and reducing its plasma levels.1 Alirocumab has been authorized by the European Medicines Agency (EMA) to treat primary hypercholesterolemia and mixed dyslipidemia as a dietary supplement through subcutaneous injections. Recently, a correlation has been suggested among cutaneous/serum levels of PCSK-9, cardiovascular risk, and severity in diseases such as psoriasis and lupus, turning it into a potential therapeutic target.2 Adverse reactions such as erythema, or pain at the injection site have been reported, being nummular eczema, urticaria, and hypersensitivity vasculitis exceptionally rare.1,3
DILEs are skin reactions that typically appear weeks, or months after starting therapy. Uncertainty still surrounds their pathophysiology to this date. Clinically and histopathologically, DILEs are almost identical to lichen planus. Therefore, a suggestive medical history, physical examination, histopathology, and a plausible course of the disease are essential for diagnostic purposes.4 A few aspects of this case supporting the diagnosis of DILE include the absence of mucosal involvement, symmetric distribution in photo-exposed areas, and residual hyperpigmentation.5
The list of drugs and vaccines associated with DILE is extensive and independent of the route of administration, including enalapril, amlodipine, beta-blockers, diuretics, antimalarials, imatinib, NSAIDs, and immunoglobulins. We should mention the existence of cases associated with simvastatin and biologic drugs such as infliximab, etanercept, pembrolizumab, and nivolumab.6,7 A search in Medline, Cochrane, and Epistemonikos databases using the keywords alirocumab, and lichenoid eruption, or lichen planus in both English and Spanish failed to reveal previously published cases.
We used the Naranjo probability scale as a tool to assess the likelihood of an association between an adverse reaction and a drug,8 which showed a “probable” association between alirocumab and DILE.
The heterogeneity reported among the different pharmacological groups, doses, and routes of administration described as possible DILE triggers suggests the presence of likely immunomediated idiosyncratic reactions, which would explain the consistent response to corticosteroid therapy in this and other cases reported.9
In conclusion, this was a case of a lichenoid eruption probably associated with the use of alirocumab, an association not described to this date.
Conflicts of interestNone declared.