We read with interest the Case and Research letter by Sardoy et al,1 and believe that it can be complemented by our interesting findings on genetic alterations in blue nevi. There are multiple clinical and histological variants of blue nevus, one of the less common of which is agminated blue nevus, which is characterized by the grouping of multiple well-defined bluish lesions.2
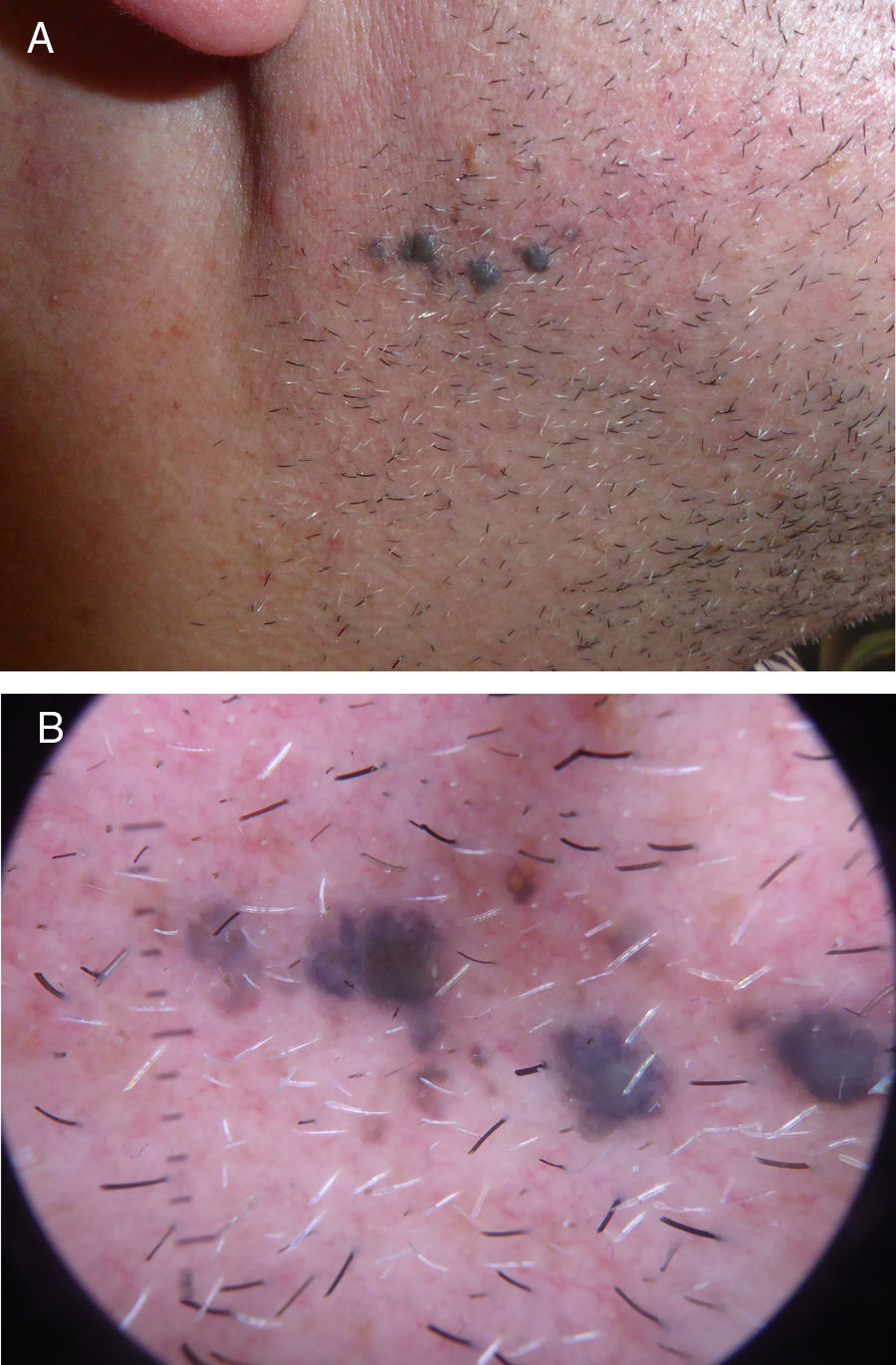
We describe the case of a 52-year-old patient with no personal or dermatological history of interest who consulted for evaluation of asymptomatic pigmented lesions in the right mandibular region that had appeared 10 years earlier and had increased in number (Fig. 1A and B). En bloc resection was performed for histological analysis (Fig. 2). Additional immunohistochemical staining showed that the sample was negative for preferentially expressed antigen in melanoma (PRAME). Ki-67 immunostaining revealed a low proliferation index. A complete sequencing study of exon 5 of GNAQ showed no alterations.
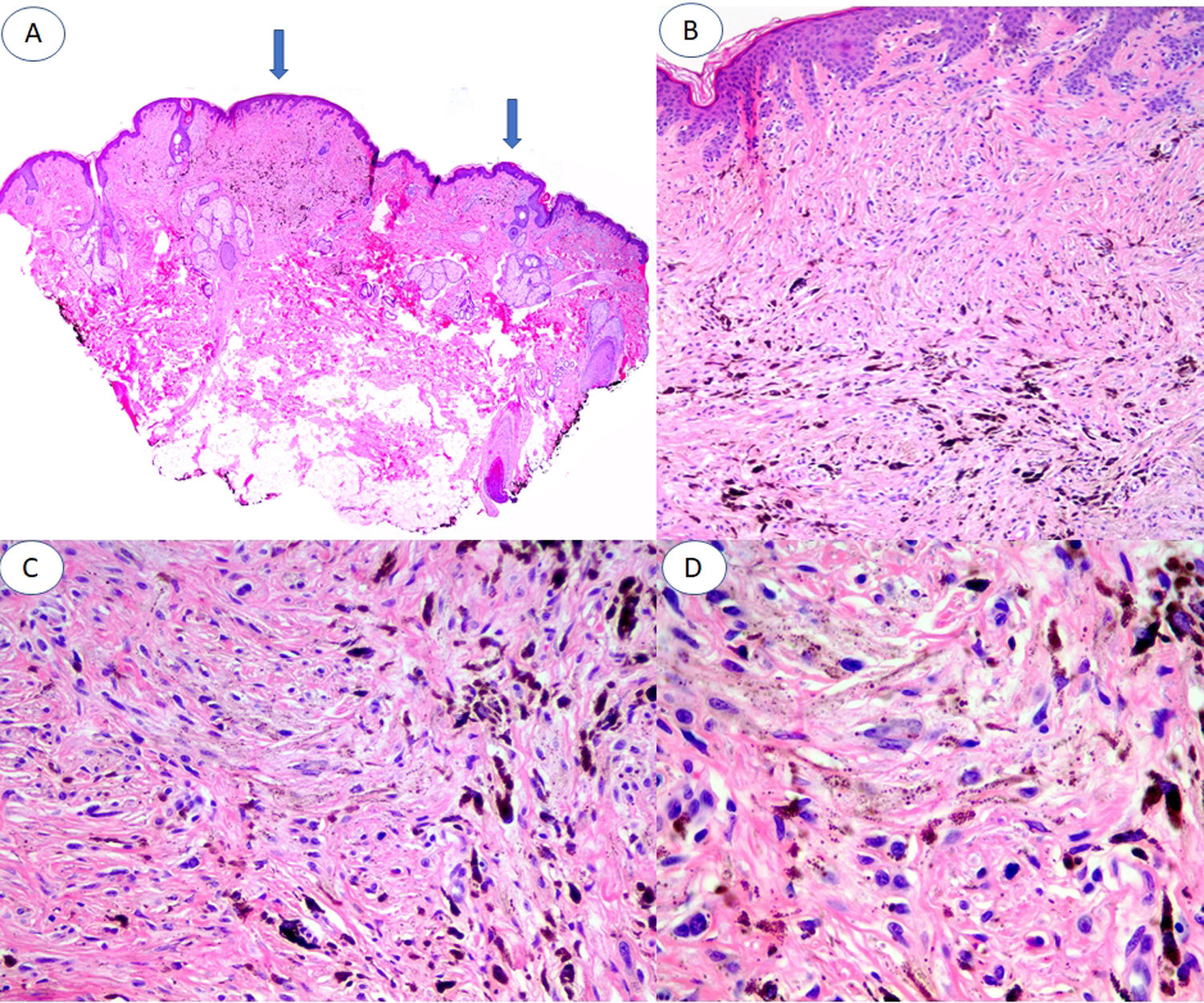
A, Panoramic image of the lesion in which 2 areas of blue nevus are evident (arrows), one of about 3 mm and the other of about 1 mm. B, Compact nests of spindle-shaped melanocytes located in the papillary and superficial reticular dermis without the presence of nests at the dermoepidermal junction (hematoxylin-eosin [HE], original magnification ×4). C, Detail of melanin distribution, with denser granules visible in the deepest part of the lesion (HE, original magnification ×10). D, Detail of melanocytes, showing monomorphic nuclei and the absence of mitosis and necrosis (HE, original magnification ×20).
Based on these clinical and histological data, diagnosis of agminate blue nevus was established.
This infrequent presentation of blue nevus is of interest given the potential for clinical diagnostic confusion with melanoma metastasis. Genetic alterations potentially shared with uveal melanoma, nevus of Ota, and nevus of Ito are of particular interest.3–7
In blue nevus the most frequently described genetic mutation, which is found in up to 83% of cases and considered a driver mutation, is a somatic mutation in GNAQ and GNA11 (which encode the alpha subunits of heterotrimeric G proteins involved in G-protein-coupled receptor-mediated signaling). This mutation causes the affected genes to act as oncogenes, as it results in constitutive activation of these proteins and continuous activation of the Ras signaling pathway, which is involved in regulation of the cell cycle and proliferation.3 Consequently, in blue nevi with this mutation there is an increase in the activity of mitogen-activated protein kinase 1 (ERK2), which is activated by RAS/RAF/MAP kinase kinase (MEK).
There is only 1 published report of agminate blue nevus with a proven mutation, located at c.626A > T (p.Glu209Leu) in GNAQ.8
In some reported cases, including ours, in which no GNAQ mutation is detected, activating mutations have been detected in cysteinyl leukotriene receptor 2 (CYSLTR2), which is involved in a signaling pathway analogous to that of GNAQ and ultimately activates the same intracellular processes. This is yet another driver mutation, mutually exclusive from GNAQ.6
Driver mutations affecting GNAQ/GNA11 and CYSLTR2 can be accompanied by additional mutations in different cellular pathways that favor the progression and malignant transformation of the lesions.4–7 The most widely known mutation is in the BRCA1-associated protein 1 gene (BAP1). BAP1 acts as a tumor suppressor and is involved in DNA repair processes, the ubiquitin-proteasome system, regulation of transcription, and chromatin modulation.5 Inactivation of this protein, either by partial deletion of the BAP1 locus on chromosome 3 or alteration in the phosphorylation or ubiquitination of its protein chain, results in accelerated progression of cell proliferation that can trigger malignant transformation of blue nevus.5
Furthermore, these alterations described for blue nevus and for blue-nevus-like melanoma closely resemble those found in other pathological processes such as uveal melanoma. For example, the GNAQ mutation has been described in 46% of uveal melanomas, where it also acts as a driver mutation and can be associated with other mutations affecting BAP1 or other pathways involved in chromosomal instability.5
Knowledge of genetic alterations and intracellular signaling pathways in these lesions is not only of diagnostic value, but may also have therapeutic implications. For example, the use of MEK inhibitors, with or without phosphatidyl-inositol 3 kinase (PI3K) or mammalian target of rapamycin (mTOR) inhibitors, has been proposed for the treatment of uveal melanoma and could also prove useful for the treatment of blue nevi with progression to melanoma.4
Finally, PRAME immunostaining has been recently proposed as a diagnostically useful marker to distinguish between nevi and melanoma, and is a biomarker of metastatic risk in uveal melanoma.9,10 In our case, the negative PRAME immunostaining is in line with the other histological findings that support a diagnosis of blue nevus in our patient. To our knowledge, this is the first report of PRAME staining of agminate blue nevus.
Given the potential diagnostic and therapeutic implications of blue nevus with an atypical clinical presentation, as in the present case of agminate blue nevus, knowledge of the underlying genetics is important. PRAME immunohistochemistry and genetic analyses can increase diagnostic specificity in cases of atypical melanocytic lesions.
Conflicts of interestDr. Llamas has acted as a speaker and consultant for Janssen-Cilag, AbbVie, Celgene, Pfizer, Novartis, Lilly, Almirall, and Leo-Pharma, and has participated in clinical trials. The remaining authors have no conflicts of interest to declare.
The authors thank the entire Friedrichshafen team for their unconditional support, as well as Dr. Gabriele Palmero, Dr. Maximiliano Aragües, and Dr. Javier Fraga.
Please cite this article as: Rodríguez-Jiménez P, Mayor-Sanabria F, Rütten A, Fraga J, Llamas-Velasco M. Nevus azul agminado, mutaciones en GNAQ y más allá. Actas Dermosifiliogr. 2021;112:95–97.

![A, Panoramic image of the lesion in which 2 areas of blue nevus are evident (arrows), one of about 3 mm and the other of about 1 mm. B, Compact nests of spindle-shaped melanocytes located in the papillary and superficial reticular dermis without the presence of nests at the dermoepidermal junction (hematoxylin-eosin [HE], original magnification ×4). C, Detail of melanin distribution, with denser granules visible in the deepest part of the lesion (HE, original magnification ×10). D, Detail of melanocytes, showing monomorphic nuclei and the absence of mitosis and necrosis (HE, original magnification ×20). A, Panoramic image of the lesion in which 2 areas of blue nevus are evident (arrows), one of about 3 mm and the other of about 1 mm. B, Compact nests of spindle-shaped melanocytes located in the papillary and superficial reticular dermis without the presence of nests at the dermoepidermal junction (hematoxylin-eosin [HE], original magnification ×4). C, Detail of melanin distribution, with denser granules visible in the deepest part of the lesion (HE, original magnification ×10). D, Detail of melanocytes, showing monomorphic nuclei and the absence of mitosis and necrosis (HE, original magnification ×20).](https://static.elsevier.es/multimedia/15782190/0000011200000001/v1_202101161046/S1578219020303735/v1_202101161046/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)



