The increasingly widespread use of programmed death (PD) 1 and PD ligand (PD-L1) inhibitors to treat cancer has led to growing interest in the need for a detailed knowledge and description of their adverse effects.1,2 Dermatologic adverse effects are the earliest to appear and the most common. While it is known that these effects are caused by activation of T lymphocytes resulting from blockade of the PD-1 receptors and the ligand PD-L1, we still know little of the mechanism underlying the abnormal targeting of dermal-epidermal antigens by immune cells.3,4 Below, we report a series that brings together experience on cutaneous adverse effects associated with these drugs in a reference center.
We selected all patients included in the registry of the Pharmacy Department of Hospital General Universitario de Valencia, Valencia, Spain who had received immune checkpoint inhibitors between 2014 and 2021. The series comprised 313 patients, of whom 10 had received treatment with durvalumab, 139 with pembrolizumab, 112 with nivolumab, 51 with atezolizumab, and 1 with avelumab.
We identified patients whose skin abnormalities could be attributed to the adverse effects of PD-1 or PD-L1 inhibitors. We then collected relevant data to characterize the dermatologic abnormalities found and determined the time between initiation of treatment with immune checkpoint inhibitors and onset. The adverse effects were classified as follows: alopecia, eczema, erythema nodosum, granuloma annulare, lichenoid reactions or lichen planus, pemphigoid, pruritus, psoriasis, palmoplantar pustulosis, sarcoidosis, toxicoderma, vitiligo, and xerosis. We also obtained information on the primary tumor owing to its possible relevance in the onset of adverse effects.
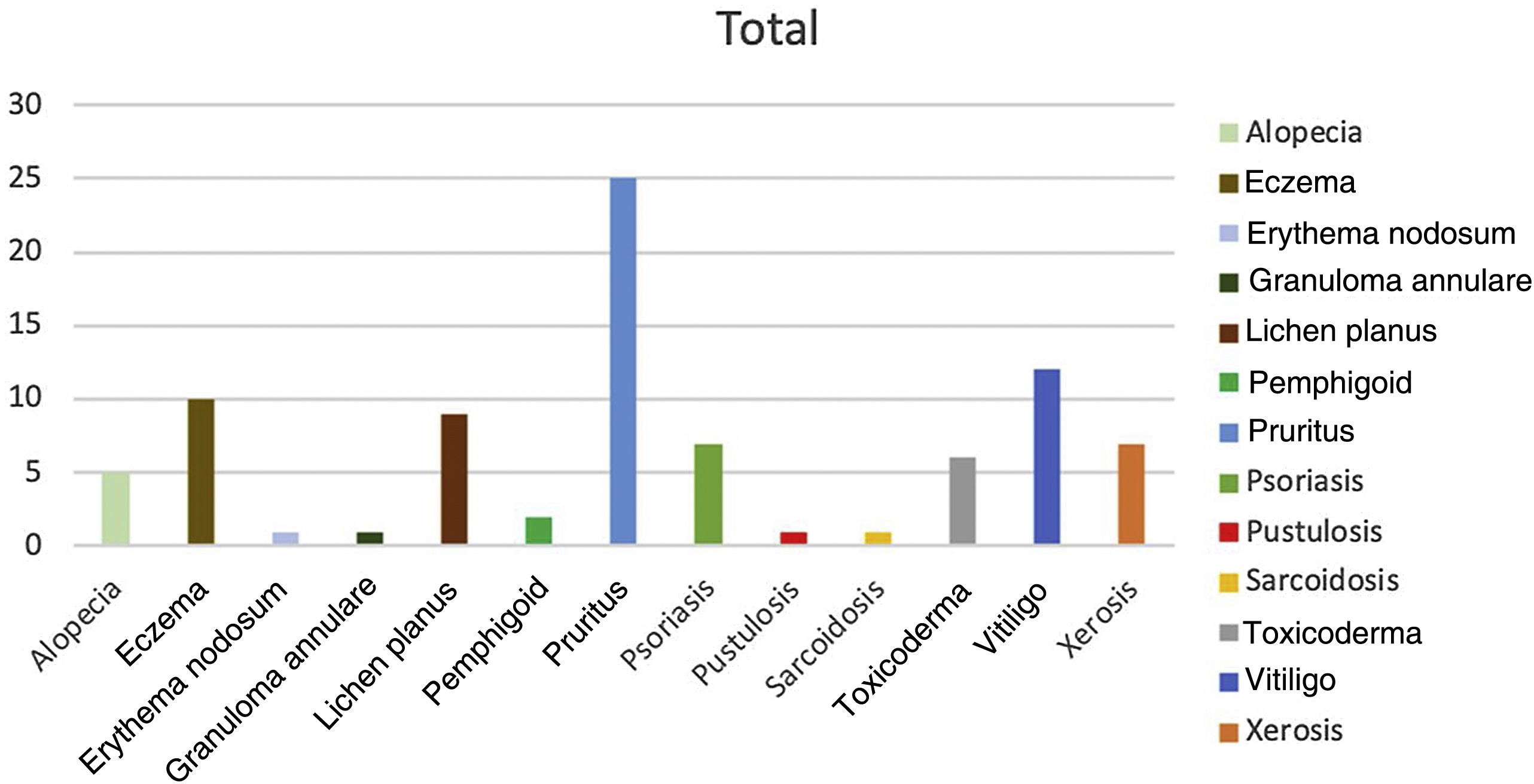
Of the 313 patients analyzed, 40 (12%) had adverse reactions to PD-1 or PD-L1 inhibitors. The highest number of adverse effects was recorded with nivolumab (incidence of 16%), followed by pembrolizumab (14%). Of the 51 patients who received atezolizumab exclusively, only 1 experienced an adverse effect. A significantly lower number of dermatologic adverse effects were recorded in patients receiving PD-L1 inhibitors (incidence, 3.2%) than in patients receiving PD-1 inhibitors (15.1%) (P<.05, χ2). All the adverse effects responded favorably to topical and/or systemic treatments, with no need to modify or suspend immunotherapy in any cases. The highest incidence was recorded for melanoma, with adverse effects in 40.8%, compared to only 6.7% in patients with lung cancer. Only 1 adverse effect was detected in 30% of patients; more than 1 effect was recorded in the remaining 70%. Of note, 2 patients developed 6 effects: in both cases the primary tumor was melanoma. The most common cutaneous reaction in the series was pruritus (n=25), followed by vitiligo (n=12) and eczema (n=10) (Fig. 1).
The present study made it possible for us to establish an association between the use of PD-1 and PD-L1 inhibitors and the onset of dermatologic adverse effects. The most frequent effects were pruritus, vitiligo, and eczema, as reported elsewhere.5 The size of our sample enabled us to detect effects that are less common, such as sarcoidosis, pustulosis, and bullous pemphigoid.6,7
In the series we studied, we were able to associate the incidence of adverse effects with the type of primary tumor, irrespective of the drug administered. This was particularly true for melanoma, where adverse effects were observed in 40% of affected patients; this incidence was higher than in other studies. However, we cannot rule out selection bias, since patients with melanoma have regular checkups in the dermatology department; therefore, adverse effects affecting the skin and adnexa are more likely to be detected. Furthermore, vitiligo, which is one of the most frequent adverse effects of PD-1 and PD-L1 inhibitors,8 almost exclusively affects patients with melanoma, thus potentially explaining the greater incidence of adverse effects than in patients with other primary tumors.
The onset of adverse effects has been associated with greater survival of affected patients.9,10 This could be attributed to more potent activation of the immune system, which acts against both tumor cells and healthy tissue. The adverse effects observed were tolerable. Systemic treatment was necessary in more severe manifestations (6 patients with intense pruritus and sleep disturbances, 1 patient with generalized eczema, 1 patient with extensive lichen planus, 2 patients with bullous pemphigoid, and 1 patient with sarcoidosis), although it was not necessary to suspend immunotherapy in any of them. We must identify and control these adverse effects early so that they do not lead to interruption of treatment.
Conflicts of InterestThe authors declare that they have no conflicts of interest.