Biologic therapy is widely used in the treatment of psoriasis, and the incidence of adverse cutaneous effects induced by tumor necrosis factor (TNF) α antagonists is increasing.1 A wide range of skin lesions, with variable morphologic features and causes, have been reported in addition to injection site reactions and skin infections. Of particular note is the increasing number of reports of immune-mediated paradoxical reactions, such as psoriasis and psoriasis-like rashes.2 Only 5 cases of lichenoid reactions associated with TNF-α antagonists, however, have been reported to date.
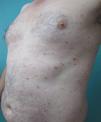
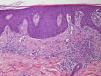
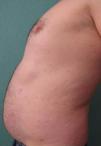
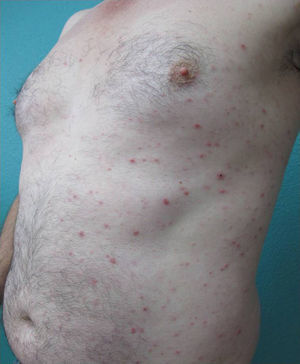
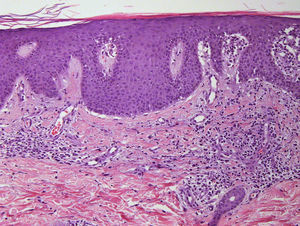
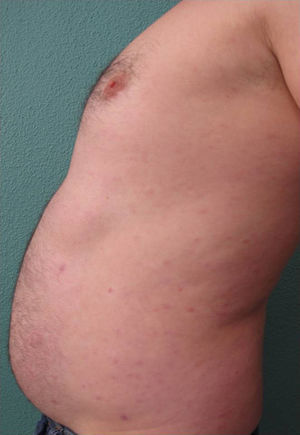
We present the case of a 45-year-old hypertensive, diabetic man, with no known allergies, who had a history of unstable moderate to severe plaque psoriasis of 20 years’ duration associated with psoriatic arthritis of 3 years’ duration. The patient had been treated with topical drugs (various), systemic therapy (oral acitretin), and subsequently, biologic therapy (etanercept—interrupted at 15 months due to a loss of effectiveness—and ustekinumab—interrupted at 9 months due to lack of indication for use in psoriatic arthritis at the time of diagnosis). The patient, with a Psoriasis Area and Severity Index (PASI) score of 12 and a body surface area score of 18, eventually continued treatment with adalimumab 40mg, and achieved PASI 0 at 8 weeks, with resolution of joint pain. Shortly afterwards, he reported the appearance of an intermittent rash consisting of small asymptomatic lesions that did not coincide with the presence of infections or fever or the use of other medications. The rashes had been believed to be outbreaks of guttate psoriasis for a period of 8 months, and did not resolve on interruption of adalimumab therapy. Examination revealed multiple, small, firm scaling erythematous papules that covered large areas of the trunk and the proximal extremities, with sparing of the palms, soles, and mucous membranes. The lesions were at different stages of development; some were residual pigmented lesions, while others had a lichenoid appearance with thin, superficial micaceous scales (Fig. 1). The diagnosis was revisited, and laboratory tests, including complete blood count, biochemistry, serology, antistreptolysin O, and antinuclear antibodies, were ordered. All the results were negative or within normal ranges. Skin biopsy showed psoriasiform acanthosis with minimal parakeratosis, a discrete perivascular lymphohistiocytic inflammatory infiltrate and extravasated red blood cells, and isolated vacuolar changes in the basal layer, in addition to minimal epidermal lymphocytic exocytosis and isolated necrotic keratinocytes (Fig. 2). These findings were consistent with a diagnosis of pityriasis lichenoides chronica (PLC). It was decided to add methotrexate 15mg weekly to the existing treatment with adalimumab. The rash resolved at 6 weeks, and the methotrexate dose was subsequently reduced, and the treatment withdrawn at 10 weeks. Three months later, the patient reported new lesions, which were again brought under control with methotrexate. After 6 months of follow-up, the patient remains asymptomatic (Fig. 3).
Many adverse skin reactions have been reported in association with TNF-α antagonists.3 In a recent publication, Newell et al.4 reported the development of PLC in a patient with psoriasis after the third infusion of infliximab, and in a later study, Said et al.5 published 2 cases of PLC in patients with Crohn disease under treatment with adalimumab. In both cases, complete remission was achieved following the addition of methotrexate. López-Ferrer et al.6 also described a case of PLC after the use of infliximab in a patient with ankylosing spondylitis and ulcerative colitis. A favorable response was also observed when methotrexate was added. Finally, Echeverri et al.7 published a case of PLC that appeared during week 6 of etanercept therapy in a patient with rheumatoid arthritis. The condition improved with the use of topical corticosteroids. Our case is the sixth report in the literature of PLC induced by a TNF-α antagonist and the first of good response to methotrexate in a patient with psoriasis being treated with adalimumab. Although we cannot rule out spontaneous resolution, our experience is similar to that reported by Said et al. and López-Ferrer et al. What causes pityriasis lichenoid and its variants remains unknown,8 but TNF-α appears to have a role, as increased serum levels of this cytokine have been reported in a patient with febrile ulceronecrotic Mucha-Habermann disease9 and etanercept was also found to be effective in a case of pityriasis lichenoides that was unresponsive to multiple treatments.10 In our opinion, the development of PLC during treatment with TNF-α antagonists can also be considered a paradoxical reaction related to this relationship, just like the multiple cases of psoriasiform reactions and palmoplantar pustulosis reported over the last decade.11 Although the pathogenesis and etiology of these reactions are unknown, the most widely accepted theory is an imbalance of cytokines, which would trigger the compensatory production of interferon and the development of paradoxical reactions in genetically predisposed individuals.
In conclusion, adverse cutaneous effects associated with the use of TNF-α antagonists are more common than originally estimated, and the development of immune-mediated diseases in this setting is gaining recognition as an emerging phenomenon. We have described a new case of PLC associated with adalimumab and have shown how methotrexate is an interesting treatment option due to the synergic action between this drug and TNF-α antagonists in psoriasis.
Please cite this article as: Martínez-Peinado C, Galán-Gutiérrez M, Ruiz-Villaverde R, Solorzano-Mariscal R. Pitiriasis liquenoide crónica inducida por adalimumab en paciente con psoriasis y buena respuesta a metotrexato. Actas Dermosifiliogr. 2016;107:167–169.