To the Editor:
Hypertrichosis is defined as the excessive growth of hair on any part of the body.1 In hirsutism, on the other hand, the hair follicles have different characteristics and an androgenic distribution. Clinically, hypertrichosis is classified according to its extension, as localized or generalized, and to its etiology, as congenital or acquired. The origin of acquired generalized hypertrichosis can be idiopathic, iatrogenic, or related to systemic diseases,2 such as hypothyroidism, porphyria, celiac disease, dermatomyositis, human immunodeficiency virus (HIV) infection, or tumors. The widespread appearance of lanugo in the adult may be a sign of internal cancer or portend the onset of malignant disease; however, there have been no reports of such cases in children.3
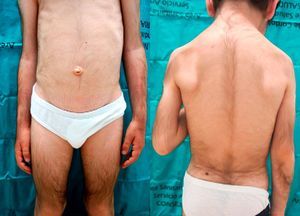
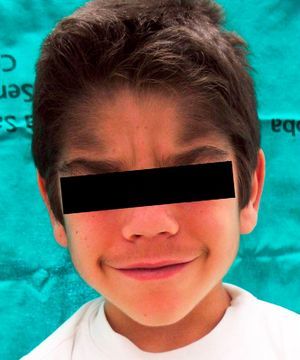
We report the case of a 6-year-old boy who was referred to our outpatient clinic for the growth of terminal hair that had started to develop on the face, trunk, and limbs (Fig. 1) during his first year of life and had been slowly progressive. The patient had been diagnosed with hyperinsulinemic hypoglycemia of infancy at 5 months of age and was on treatment with diazoxide, 20mg/kg/d, with adequate control. There was no family history of interest. He did not present growth retardation, neurological disturbances, or other typically relevant underlying diseases or treatments. Physical examination revealed widespread hypertrichosis, most marked on the forehead, upper lip, and eyebrows, which had started to develop some months after introduction of the full dose of diazoxide (Fig. 2). There was no predilection for sun-exposed areas. No other lesions were observed on the skin or mucosas and his teeth were normal. Blood tests including a complete blood count, routine biochemistry, thyroid hormones, and lactate dehydrogenase as well as antigliadin, antiendomysium, and antitransglutaminase antibodies were normal. HIV serology was negative. A diagnosis of acquired generalized hypertrichosis secondary to treatment with diazoxide was made on the basis of the above findings.
Iatrogenic acquired generalized hypertrichosis preferentially affects the temporal and frontal regions, the flexor surfaces of the limbs, and the trunk and is usually reversible after withdrawal of the causative agent. The drugs most frequently implicated are ciclosporin, phenytoin, and minoxidil. Diazoxide is an uncommon cause of generalized hypertrichosis,4 which develops in 1% to 20% of adults receiving this treatment. In children, however, this figure may reach 100%. This adverse effect appears to be related to the dose and to the duration of treatment. In adults, diazoxide is principally used to control episodes of malignant hypertension, whereas the most common indication in children is hyperinsulinemic hypoglycemia of infancy,5 in which it is used for maintenance treatment. This would explain the higher incidence of generalized hypertrichosis in children. The pathogenesis is thought to be related to activation of the ATP-dependent potassium channels. These channels are present both on pancreatic cells and on cells in the hair follicles. A recent publication has described how drugs that act on these channels affect the anagen (growth) phase of hair follicles6: those that block the potassium channels, such as tolbutamide, shorten the anagen phase whereas agonists, such as minoxidil and diazoxide, lengthen this phase.7 Treatment is limited to cosmetic hair removal with topical agents or the use of photoepilation systems. The application of 11.5% eflornitine cream has been shown to be effective in the treatment of facial hirsutism and has been used off-label in patients with facial hypertrichosis.
In view of these findings, studies must be performed in any child presenting generalized hypertrichosis to exclude the systemic diseases listed above and to look for possible causative drugs; if such a drug is detected, the risks and benefits of changing or withdrawing the treatment must be evaluated.
Please cite this article: Salido R, et al. Hipertricosis generalizada adquirida por diazóxido. Actas Dermosifiliogr. 2013;104:166–7.