Dermatitis herpetiformis is a chronic bullous disease that is currently considered a cutaneous expression of gluten hypersensitivity. The aim of this study was to analyze and describe the clinical, histological, and immunopathological characteristics of patients with dermatitis herpetiformis assessed at Hospital Clinic de Barcelona, Spain between 1995 and 2010.
Material and methodsDemographic, clinical, serologic, and histopathological data were reviewed for 33 patients with dermatitis herpetiformis.
ResultsThe median age of the patients at the time of disease onset was 30years and the majority were men. Associated autoimmune disease was present in 49% of patients. In 6 patients, celiac disease was diagnosed before dermatitis herpetiformis. Although excoriations were the most predominant lesions, 9 patients had blisters. Histological findings in skin lesions were compatible with dermatitis herpetiformis in 46% of cases. The most frequently observed staining pattern by direct immunofluorescence was the presence of granular immunoglobulin A deposits in the basement membrane (62%). More than 80% of intestinal biopsies were compatible with celiac disease. Antibodies linked to gluten sensitivity were observed in 79% of patients. Only 1 malignant tumor was detected.
ConclusionsNotable findings were the frequent presence of bullous lesions, the high prevalence of celiac disease, and the positive findings on intestinal biopsy, all of which are suggestive of late diagnosis. Our findings confirm the lack of specificity of conventional histology in dermatitis herpetiformis and the association of the disease with other immunological disorders.
La dermatitis herpetiforme (DH) es una enfermedad ampollosa crónica actualmente considerada como la expresión cutánea de la hipersensibilidad al gluten. El objetivo de este estudio es analizar y describir las características clínicas, histológicas e inmunopatológicas de los pacientes con DH valorados en el Hospital Clínic de Barcelona entre los años 1995 y 2010.
Material y métodosSe han revisado los datos demográficos, clínicos, serológicos e histopatológicos de 33 pacientes afectos de DH.
ResultadosLa mediana de edad de inicio en los pacientes estudiados fue de 30 años, con un claro predominio en el sexo masculino. El 49% presentaba algún trastorno autoinmune asociado. En 6 pacientes el diagnóstico de celiaquía precedió al de DH. Si bien las lesiones predominantes fueron las excoriaciones, 9 pacientes presentaron ampollas. El estudio histológico de las lesiones cutáneas se consideró compatible con DH en el 46% de los casos. El patrón de inmunofluorescencia directa (IFD) más frecuentemente observado fue el depósito granular de Ig A en la membrana basal (62%). Más del 80% de las biopsias intestinales fueron compatibles con enfermedad celiaca. Un 79% de los pacientes presentó anticuerpos relacionados con la sensibilidad al gluten. Sólo se detectó una neoplasia maligna.
ConclusionesDestaca la frecuente presencia de lesiones ampollosas, la elevada prevalencia de celiaquía y de positividad de la biopsia intestinal, todo ello sugestivo de un diagnóstico tardío. Nuestros datos corroboran la inespecificidad de la histología convencional en el diagnóstico de esta entidad y la asociación de la DH con otros trastornos inmunológicos.
Dermatitis herpetiformis, a chronic bullous disease described by Louis Duhring in 1884,1 mainly affects white patients. The disease may present at any age, but onset commonly occurs during the third decade of life and is rare in children. Incidence varies geographically. In Europe, prevalence estimates have ranged from 11 to 40 per 100 000 population.2,3 The family incidence has been estimated at between 2.3% and 6.5%.4,5
The course of this disease encompasses episodes of intensely pruritic polymorphic skin lesions found mainly on the elbows, knees, buttocks, and scalp.
Histologic findings are nonspecific in 37% of the cases and change as the lesions develop. In new lesions, polymorphonuclear cells can be observed in dermal papillae, the superficial dermis contains inflammatory infiltrates in varying degrees of intensity, and subepidermal bullae may form.6 In late stages, only vascular ectasia and fibrosis will be present.
A firm diagnosis requires a finding of granular immunoglobulin (Ig) A deposits in the basement membrane on direct immunofluorescence (DIF).6
Intestinal disease in association with dermatitis herpetiformis, reported by Marks and coworkers,7 was later found to be a gluten-sensitive enteropathy leading to digestive symptoms and skin lesions that responded to a gluten-free diet.8 In 1983 the presence of IgA class antiendomysial (AEM) antibodies was reported in a large percentage of patients (52%-100%) with dermatitis herpetiformis.9 Furthermore, antibodies directed at tissue transglutaminase (TGt) were found to correlate directly with damage to the intestinal mucosa; TGt titers fell with a gluten-free diet.10
Although all patients with dermatitis herpetiformis have gluten sensitivity, only between 3% and 10% of patients with celiac disease will develop the skin condition during their lifetime. Both diseases are multifactorial in origin, arising from environmental and genetic causes, and a strong association with the human leukocyte antigens (HLA) DQ2 (80%-90%) and DQ8 (10%-20%) has been confirmed.11 Dermatitis herpetiformis is currently considered to be a skin manifestation of gluten sensitivity.12
In dermatitis herpetiformis, an autoimmune disease characterized by the formation of subepidermal bullae, antibodies do not target any component of the dermoepidermal junction. Sardy and coworkers11 recently showed that the epidermal transglutaminase enzyme (TGe), which is a homologue of TGt but not identical to it, is the predominant autoantigen in patients with this diagnosis.
Other autoimmune disorders have been described in association with dermatitis herpetiformis,13,14 and there are reports of increased risk of lymphoma15 and other tumors.16 The link to malignancy, however, is disputed.17
Our aim in this study was to analyze and describe the clinical, histologic, and immunopathologic features of dermatitis herpetiformis in patients treated in Hospital Clínic de Barcelona between 1995 and 2010.
Material and MethodsClinical DataAll cases of dermatitis herpetiformis on record in Hospital Clínic de Barcelona from 1995 to 2010 (15 years) were reviewed retrospectively. A positive DIF finding was essential for a firm diagnosis, allowing us to rule out dermatitis herpetiformis in patients with negative DIF results even if clinical suspicion was high. We centered our analysis on the features of skin lesions, age at onset, time between first symptoms and diagnosis, family and personal histories of celiac disease or other autoimmune diseases or tumors, and associated digestive symptoms.
Histopathologic FindingsThe skin biopsies for these patients had been paraffin embedded and sectioned and then stained with hematoxylin-eosin.
The observation of polymorphonuclear cells in dermal papillae, whether accompanied by dermoepidermal separation or not, was considered compatible with a diagnosis of dermatitis herpetiformis. Biopsy tissues without such findings were considered nonspecific.
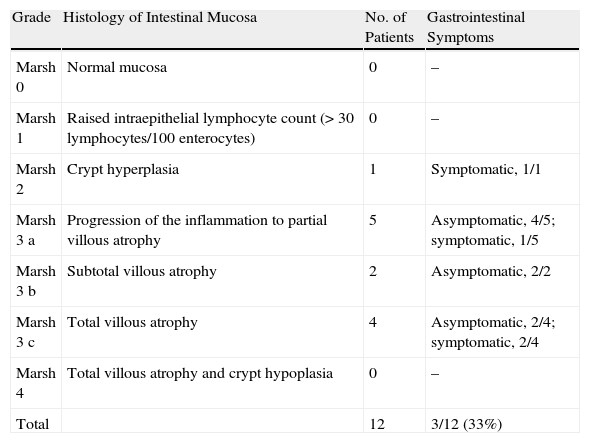
The Marsh classification was used to categorize histologic findings in biopsies of intestinal mucosa according to the severity of lesions (Table 1).18
Marsh Classification of Intestinal Disease and Symptoms.
| Grade | Histology of Intestinal Mucosa | No. of Patients | Gastrointestinal Symptoms |
| Marsh 0 | Normal mucosa | 0 | – |
| Marsh 1 | Raised intraepithelial lymphocyte count (> 30 lymphocytes/100 enterocytes) | 0 | – |
| Marsh 2 | Crypt hyperplasia | 1 | Symptomatic, 1/1 |
| Marsh 3 a | Progression of the inflammation to partial villous atrophy | 5 | Asymptomatic, 4/5; symptomatic, 1/5 |
| Marsh 3 b | Subtotal villous atrophy | 2 | Asymptomatic, 2/2 |
| Marsh 3 c | Total villous atrophy | 4 | Asymptomatic, 2/4; symptomatic, 2/4 |
| Marsh 4 | Total villous atrophy and crypt hypoplasia | 0 | – |
| Total | 12 | 3/12 (33%) |
A diagnosis of dermatitis herpetiformis was confirmed by standard DIF procedures. The presence of granular deposits of IgA in the basement membrane was considered a characteristic finding. Three DIF patterns of IgA deposition were defined, as follows: a) along the basement membrane, b) in dermal papillae, and c) a mixed pattern, with deposits in both the basement membrane and dermal papillae.
SerologyEnzyme-linked immunosorbent assay was used to determine the presence of IgA antibodies related to gluten sensitivity (specifically antigliadin and anti-TGt antibodies). Indirect immunofluorescence (IIF) was used to detect the presence of AEM antibodies.
HLA TypingHLA-DQ2 and HLA-DQ8 alleles were detected by means of polymerase chain reaction techniques.
ResultsFrom 1995 through 2010, our hospital department evaluated 33 patients with dermatitis herpetiformis (11 women and 22 men). The median age at onset of skin manifestations was 30 years (40 in men, 31 in women); the range of age at onset was 9 to 72 years.
A single patient (3%) reported a family history of dermatitis herpetiformis and 5 (15%) had a family history of celiac disease. Six patients (18%) had been diagnosed with celiac disease before onset of dermatitis herpetiformis, and 10 (37% of the remaining 27 patients) reported digestive complaints (diarrhea). Intestinal biopsies from 12 of the 17 asymptomatic patients demonstrated disease in 50%.
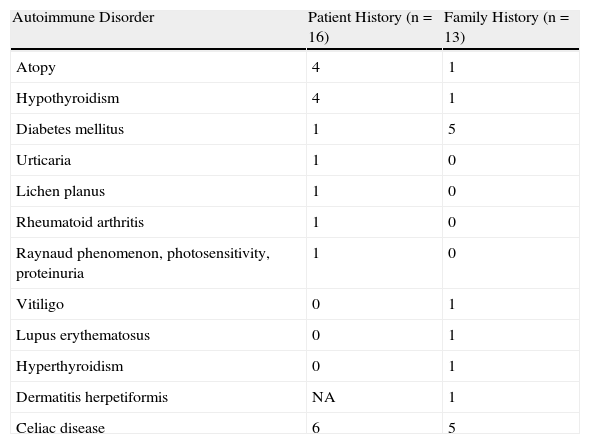
Forty-eight percent of these patients and 40% of their families had 1 or more associated autoimmune disorders (Tables 2 and 3).
Associated Autoimmune Disorders.
| Autoimmune Disorder | Patient History (n=16) | Family History (n=13) |
| Atopy | 4 | 1 |
| Hypothyroidism | 4 | 1 |
| Diabetes mellitus | 1 | 5 |
| Urticaria | 1 | 0 |
| Lichen planus | 1 | 0 |
| Rheumatoid arthritis | 1 | 0 |
| Raynaud phenomenon, photosensitivity, proteinuria | 1 | 0 |
| Vitiligo | 0 | 1 |
| Lupus erythematosus | 0 | 1 |
| Hyperthyroidism | 0 | 1 |
| Dermatitis herpetiformis | NA | 1 |
| Celiac disease | 6 | 5 |
Abbreviation: NA, not applicable.
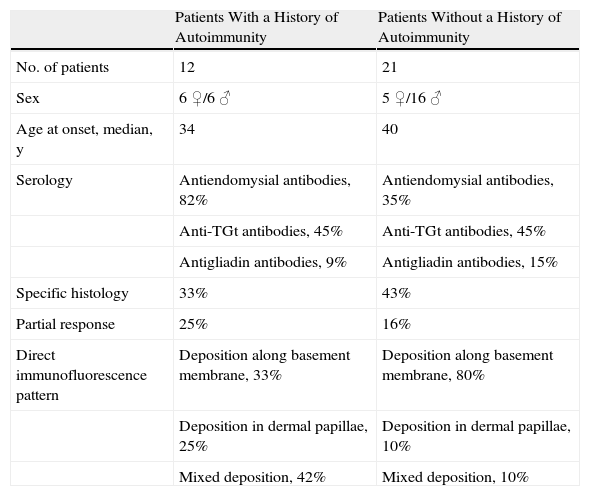
Comparison of Patients With and Without Associated Autoimmune Diseases.
| Patients With a History of Autoimmunity | Patients Without a History of Autoimmunity | |
| No. of patients | 12 | 21 |
| Sex | 6 ♀/6 ♂ | 5 ♀/16 ♂ |
| Age at onset, median, y | 34 | 40 |
| Serology | Antiendomysial antibodies, 82% | Antiendomysial antibodies, 35% |
| Anti-TGt antibodies, 45% | Anti-TGt antibodies, 45% | |
| Antigliadin antibodies, 9% | Antigliadin antibodies, 15% | |
| Specific histology | 33% | 43% |
| Partial response | 25% | 16% |
| Direct immunofluorescence pattern | Deposition along basement membrane, 33% | Deposition along basement membrane, 80% |
| Deposition in dermal papillae, 25% | Deposition in dermal papillae, 10% | |
| Mixed deposition, 42% | Mixed deposition, 10% |
Abbreviation: TGt, tissue transglutaminase.
The predominant skin lesions were described as excoriations in 12 patients; blisters in 9 patients; vesicles in 7; and scaly, erythematous, eczematous plaques in 5. Lesions were located on the elbows in 19 patients, on the forearm in 11, on the knees in 10, on the buttocks in 7, and on the back in 5. Other locations included skin folds (3 patients), the face (2), hips (2), the scalp (1), and breasts (1); 2 patients had generalized lesions.
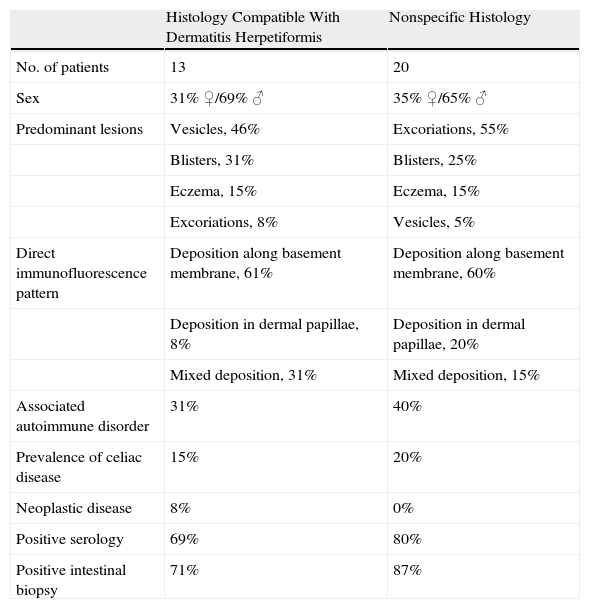
No records on skin histology were available for 5 patients. For the remaining 28 patients, histologic changes were compatible with a diagnosis of dermatitis herpetiformis. The predominant lesions in these patients were vesicles and blisters, although excoriations predominated in the patients with nonspecific histology (Table 4).
Comparison According to Findings on Skin Biopsy.
| Histology Compatible With Dermatitis Herpetiformis | Nonspecific Histology | |
| No. of patients | 13 | 20 |
| Sex | 31% ♀/69% ♂ | 35% ♀/65% ♂ |
| Predominant lesions | Vesicles, 46% | Excoriations, 55% |
| Blisters, 31% | Blisters, 25% | |
| Eczema, 15% | Eczema, 15% | |
| Excoriations, 8% | Vesicles, 5% | |
| Direct immunofluorescence pattern | Deposition along basement membrane, 61% | Deposition along basement membrane, 60% |
| Deposition in dermal papillae, 8% | Deposition in dermal papillae, 20% | |
| Mixed deposition, 31% | Mixed deposition, 15% | |
| Associated autoimmune disorder | 31% | 40% |
| Prevalence of celiac disease | 15% | 20% |
| Neoplastic disease | 8% | 0% |
| Positive serology | 69% | 80% |
| Positive intestinal biopsy | 71% | 87% |
Information on intestinal biopsy was available for 22 patients; in 18 (82%) the findings were compatible with celiac disease. Of the 16 biopsies performed in our hospital, 4 yielded no relevant information. Of the patients with a positive biopsy, 67% reported no intestinal symptoms (Table 1).
The median time between onset of skin lesions and the diagnosis of dermatitis herpetiformis was 1 year (mean, 25 months; range 1 week to 12 years). Two patients were lost to follow-up after diagnosis and the others were followed for a mean of 53 months (median, 24 months; range, 15 days to 15 years). Our department is a referral center for bullous diseases and many patients are referred only for diagnosis; the average follow-up time was therefore relatively short.
Of the 32 DIF assays performed, 20 (62%) revealed granular deposits of IgA along the basement membrane, 5 (16%) showed deposits in dermal papillae, and 7 (22%) a combination of both deposition patterns. We had no information on the DIF pattern for 1 patient for whom the procedure was done outside our hospital.
Twenty-five of the 32 patients (79%) for whom results of serology were available had positive reports at some point. Samples for 14 of the 25 contained anti-TGt antibodies (by assay available in our laboratory starting in 2007), 16 had AEM antibodies, and 4 had antigliadin antibodies. Among the patients with negative serology, 2 were following a gluten-free diet for previously diagnosed celiac disease.
HLA typing was performed for 15 patients; the HLA-DQ2 allele was present in 14 of them (93%) and the HLA-DQ8 allele was identified in 1 (7%).
A strict gluten-free diet was prescribed for all these patients (6 of whom were already following one). Sixteen patients required dapsone therapy to bring lesions under control, at least during the initial phase. In 25 of the 30 patients followed in our department, skin lesions and itching disappeared with treatment. In the cases with poor response, poor adherence to a gluten-free diet could not be ruled out.
DiscussionThe median age at diagnosis in this patient series (30 years) fell within the third decade of life. There was a clear tendency toward a higher prevalence in men (male-to-female ratio, 2:1), a pattern that is unusual for autoimmune diseases. The ratio was reversed, however, in the 6 patients under the age of 20 years at onset; in this subgroup, we saw twice the number of females (4 females, 2 males). These observations are consistent with demographic patterns reported from earlier studies.19,20
The family incidence of dermatitis herpetiformis in this series was 3%, a figure similar to others reported in the literature (2% to 5%).4,5
Eighteen percent of our patients had been previously diagnosed with celiac disease, and 5 (15%) reported the presence of that disease in the family. Little information is available on the prevalence of previously recognized celiac disease in patients with dermatitis herpetiformis, but the figures reported have usually been lower4,21 than in the series we studied.
Even though patients with dermatitis herpetiformis may be sensitive to gluten intake, they are usually asymptomatic. Histopathologic abnormalities compatible with celiac disease have been reported in 60% to 75% of patients with dermatitis herpetiformis.22 The percentage in our series was higher, at 82%, corresponding to a majority of patients with advanced enteropathy (Marsh 3), but there was no correlation between that finding and the patients’ reports of intestinal symptoms.
Although a high percentage of our patients (37%) reported digestive symptoms (diarrhea), this information is difficult to assess and might be an artifact due to a directed medical history.
Papulovesicular lesions and excoriations have classically been described as typical of dermatitis herpetiformis, along with blisters in the initial phases of disease.23
Twenty-eight percent of our patients had blisters. This finding, together with a lower prevalence of intestinal symptoms, might indicate that diagnosis was delayed in these patients, although this pattern might also reflect a phenotype that is more common in our patient population.
Histologic findings compatible with dermatitis herpetiformis were in 46% of skin biopsies; in these cases blisters and vesicles were the most frequent observations (Table 3). These results confirm that conventional histology alone lacks sensitivity and specificity for a diagnosis of dermatitis herpetiformis and underlines the need to undertake DIF when there is sufficient clinical suspicion.
Although DIF was performed in our hospital in a high percentage of patients with relatively nonspecific pruriginous lesions, in order to rule out dermatitis herpetiformis, only 2 patients with celiac disease in whom clinical suspicion was high were in fact ruled out by negative DIF results.
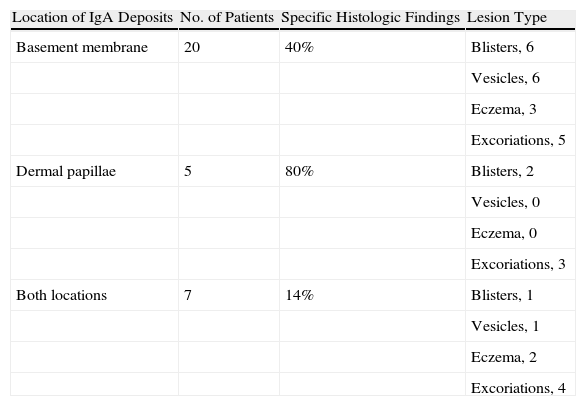
Granular IgA deposits were observed along the basement membrane in 62% of the specimens examined by DIF. We found no clear relationship between DIF pattern and patient sex or lesion location. However, in patients with high IgA deposition in dermal papillae (60%), or with a mixed pattern, the predominant lesions were more often excoriations (50%) in comparison with patients with IgA deposition in the basement membrane (25% with excoriations). While these results come from a small patient sample, they seem to suggest a relationship between IgA deposition and the presence or degree of pruritus (Table 5).
Deposition Patterns Detected by Direct Immunofluorescence.
| Location of IgA Deposits | No. of Patients | Specific Histologic Findings | Lesion Type |
| Basement membrane | 20 | 40% | Blisters, 6 |
| Vesicles, 6 | |||
| Eczema, 3 | |||
| Excoriations, 5 | |||
| Dermal papillae | 5 | 80% | Blisters, 2 |
| Vesicles, 0 | |||
| Eczema, 0 | |||
| Excoriations, 3 | |||
| Both locations | 7 | 14% | Blisters, 1 |
| Vesicles, 1 | |||
| Eczema, 2 | |||
| Excoriations, 4 |
Abbreviation: Ig, immunoglobulin.
Regarding serology, we saw no relationship between anti-TGt antibody titers and skin manifestations. We were unable to determine anti-TGe titers.
An immune-mediated mechanism was found to underlie the disease in 39% of our patients, and it was noteworthy that 12% had hypothyroidism and 3% had insulin-dependent diabetes mellitus. These figures are similar to others in the literature.4,14,24 There were more women among the patients with associated autoimmune disorders and positive results of serology (Table 3).
In addition to the relation between celiac disease and dermatitis herpetiformis, a high prevalence of atopy25 has been described. Hodgson and coworkers26 suggested that damage to the jejunal mucosa might explain this association, given that more allergens may pass through the lining in patients with gluten-sensitive enteropathy, leading to local submucosal release of IgE. Twelve percent of our patients reported events compatible with atopy.
An association between connective tissue diseases has also been documented. Systemic lupus erythematosus in particular has been observed, but other conditions have also been reported, among them polymyositis and Sjögren syndrome. A higher incidence of rheumatoid arthritis and elevated levels of rheumatoid factor have also been described in patients with dermatitis herpetiformis.27
None of our patients had been diagnosed with systemic lupus erythematosus, although 1 patient (3%) did have 3 of the diagnostic criteria for that disease. Another patient (3%) had been diagnosed with rheumatoid arthritis.
There is evidence for an association between celiac disease and chronic urticaria, as well as a positive response to a gluten-free diet.28 The possibility of looking for subclinical celiac changes in children with chronic rashes that are refractory to treatment has been suggested, but we located only a single report of urticaria in association with dermatitis herpetiformis.29 Likewise, in our series, only 1 such patient was identified.
The frequent presence of oral lesions in patients with celiac disease makes it difficult to assess the real prevalence of oral lichen planus in this population. Few authors refer to an association,30 and our observation of a single patient might be casual given the high prevalence of lichen planus lesions.
The association between lymphoma and celiac disease has been widely studied and reported; however few cases have been associated with dermatitis herpetiformis (prevalence,<2%). Most cases have been T-cell lymphomas associated with enteropathy in patients with dermatitis herpetiformis with a previous diagnosis of celiac disease. We detected no cases of lymphoma in our series and only a single case of a malignant tumor (breast adenocarcinoma). As noted earlier, the association between dermatitis herpetiformis and a tumoral process is disputed and has not been confirmed by studies that include a control group.17
The results in our records for HLA typing (93% with HLA-DQ2, 7% with HLA-DQ8) are similar to those in the literature,12 in spite of the small subsample of only 15 patients typed in our series.
A gluten-free diet is considered essential for control of celiac disease. However, traces of gluten protein are inevitably present in some foods. A prospective trial by Catassi and coworkers31 established 50mg/d as the threshold of tolerance for gluten intake in celiac patients.
ConclusionsWe have analyzed the demographic and clinical characteristics as well as the findings of pathology and serology for a series of 33 patients with dermatitis herpetiformis diagnosed in our hospital between 1995 and 2010.
The patient characteristics for this series are comparable to those described in the literature.
Blisters were often observed, the prevalence of celiac disease was high, and changes in intestinal mucosa were often demonstrated on biopsy. Our observations may be related to the stage of disease at the time these patients were evaluated: some may have reached a more advanced stage.
This analysis confirmed conventional histology's lack of sensitivity for a diagnosis of dermatitis herpetiformis and the need to perform confirmatory DIF in situations of strong clinical suspicion.
Specific histology compatible with a diagnosis of dermatitis herpetiformis was most often found in patients with IgA deposition in dermal papillae (80%) (Table 5). However, an analysis of lesion type in this same group of patients showed a predominance of excoriations, a finding which would be expected to correlate with a nonspecific histology. We cannot explain this inconsistency given that our series is small and we have not found studies that analyze the relation between histologic pattern on DIF and skin manifestations.
We detected a high prevalence of immunologic disorders, confirming the relationship between dermatitis herpetiformis and thyroiditis, insulin-dependent diabetes mellitus, rheumatoid arthritis, and chronic urticaria. We also noted a considerable presence of atopic dermatitis, suggesting an association between the 2 types of skin condition.
The incidence of lymphomas or other malignant tumors was very low in this series. However, the short period of follow-up (median, 24 months) does not allow us either to confirm or rule out an association.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Fuertes I, et al. Estudio retrospective de las características clínicas, histológicas e inmunológicas de los pacientes con dermatitis herpetiforme. Experiencia del Hospital Clínic de Barcelona entre los años 1995 y 2010 y revisión de la literatura. Actas Dermosifiliogr. 2011;102:699-705.