Biologic therapy has been a step forward in the control of moderate and severe psoriasis. However, major issues remain, including a better understanding of the risks associated with the use of this therapy during pregnancy. This information is usually obtained from the description of cases of accidental exposure in clinical trials, in observational studies, in clinical practice, or in patient registers such as BIOBADADERM, the methodology of which has been described previously. Based on a review of cases in BIOBADADERM and in the literature, our aim has been to define the risk of exposure to biologic agents during pregnancy.
In addition to the data in BIOBADADERM, specific information was gathered on the presence or absence of fetal abnormalities. The estimated duration of fetal exposure was calculated using the date of the final dose administered and the date of the last menstrual period.
The search for studies to review was performed on the Medline (via OVID) and Embase databases up to March 2016, with no language limits, combining 3 groups of terms: (psoriasis) AND (pregnancy) AND (infliximab, etanercept, adalimumab, ustekinumab, tumor necrosis factor-alpha/adverse effects, tumor necrosis factor-alpha/antagonists and inhibitors, tumor necrosis factor-alpha/contraindications, tumor necrosis factor-alpha/drug effects and interleukin-12,23 p40 subunit/antagonists and inhibitors). All terms were used as MeSH and as free terms
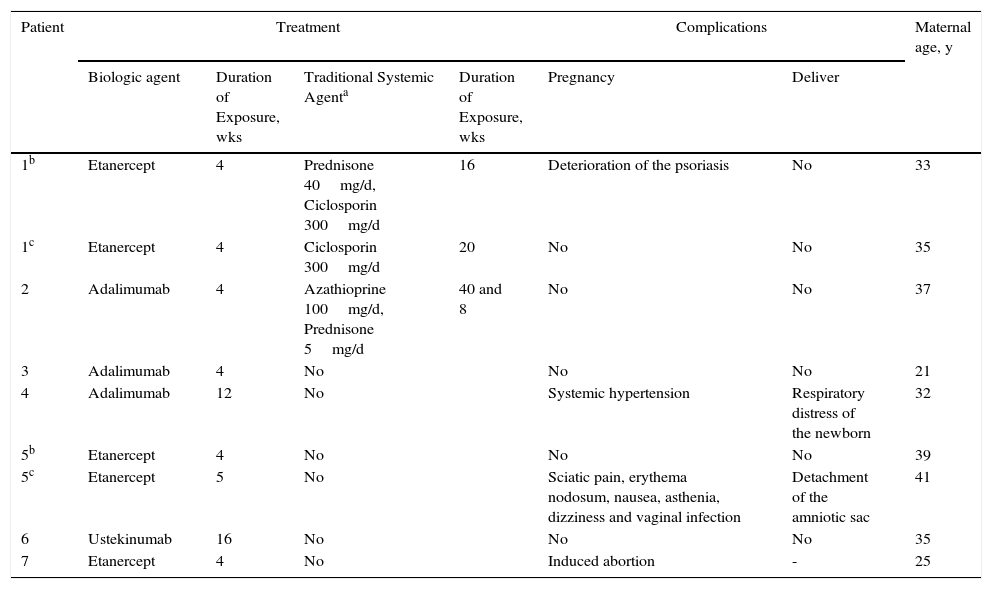
We present 7 cases of patients with moderate or severe psoriasis directly exposed to biologic therapy either during gestation or at conception as accidental exposure to the drug (Table 1). Two of the patients had 2 pregnancies with healthy children.
Pregnancies Recorded in the BIOBADADERM Register.
| Patient | Treatment | Complications | Maternal age, y | ||||
|---|---|---|---|---|---|---|---|
| Biologic agent | Duration of Exposure, wks | Traditional Systemic Agenta | Duration of Exposure, wks | Pregnancy | Deliver | ||
| 1b | Etanercept | 4 | Prednisone 40mg/d, Ciclosporin 300mg/d | 16 | Deterioration of the psoriasis | No | 33 |
| 1c | Etanercept | 4 | Ciclosporin 300mg/d | 20 | No | No | 35 |
| 2 | Adalimumab | 4 | Azathioprine 100mg/d, Prednisone 5mg/d | 40 and 8 | No | No | 37 |
| 3 | Adalimumab | 4 | No | No | No | 21 | |
| 4 | Adalimumab | 12 | No | Systemic hypertension | Respiratory distress of the newborn | 32 | |
| 5b | Etanercept | 4 | No | No | No | 39 | |
| 5c | Etanercept | 5 | No | Sciatic pain, erythema nodosum, nausea, asthenia, dizziness and vaginal infection | Detachment of the amniotic sac | 41 | |
| 6 | Ustekinumab | 16 | No | No | No | 35 | |
| 7 | Etanercept | 4 | No | Induced abortion | - | 25 | |
In patients 1A, 1B, and 2, the biologic was administered during the first weeks of pregnancy and was discontinued when the situation became known. Deterioration of the patient's psoriasis during the second or third trimester of pregnancy subsequently made it necessary to administer treatment with traditional systemic drugs.
Complications or adverse events were recorded during the pregnancy in 3 of the 7 patients: deterioration of the psoriasis (case 1A); systemic hypertension at the first obstetric appointment (case 4); and sciatic pain, erythema nodosum, nausea, fatigue, dizziness, and vaginal infection (case 5B). Voluntary interruption of pregnancy was performed in a patient exposed to etanercept for 4 weeks and with a previous daughter with Down syndrome (case 7). Complications during labor were reported in 2 of the pregnancies carried to term: respiratory distress in the newborn (case 4) and detachment of the amniotic sac (case 5B) (Table 1). No fetal abnormalities were observed in either case.
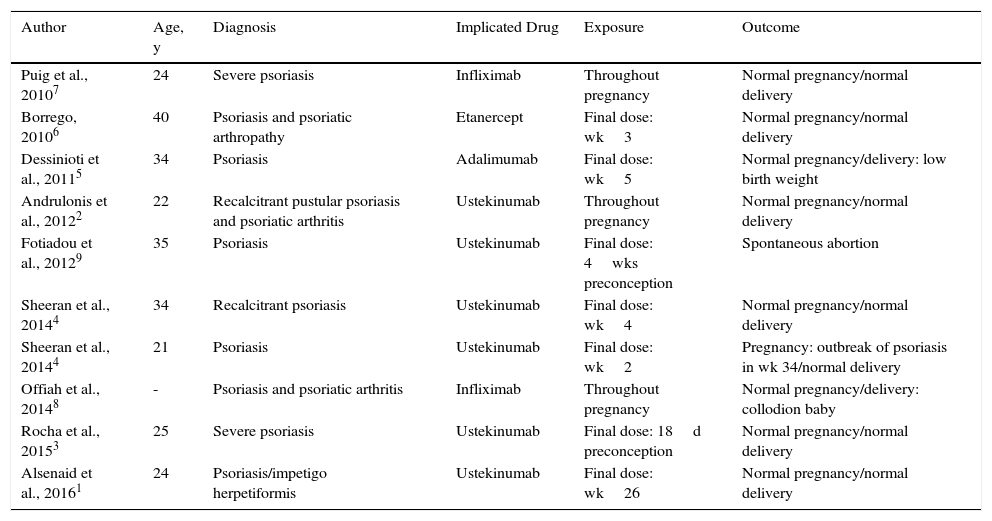
The results found in the literature are presented in Table 2. Ten patients with psoriasis were treated with biologic therapy during gestation. Six of these patients received ustekinumab: 1 with impetigo herpetiformis was treated up to week 26,1 1 with recalcitrant pustular psoriasis and psoriatic arthritis received treatment for practically her whole pregnancy,2 and the remaining 4 received ustekinumab accidentally during the first weeks of pregnancy.3,4 One of the patients was treated with adalimumab5 and another with etanercept6 during the initial weeks of pregnancy, and 2 patients were prescribed treatment with infliximab throughout pregnancy.7,8 A normal pregnancy and delivery were observed in all but 2 patients: 1 with a spontaneous abortion,9 and 1 with collodion baby.8
Review of Cases in the Literature.
| Author | Age, y | Diagnosis | Implicated Drug | Exposure | Outcome |
|---|---|---|---|---|---|
| Puig et al., 20107 | 24 | Severe psoriasis | Infliximab | Throughout pregnancy | Normal pregnancy/normal delivery |
| Borrego, 20106 | 40 | Psoriasis and psoriatic arthropathy | Etanercept | Final dose: wk3 | Normal pregnancy/normal delivery |
| Dessinioti et al., 20115 | 34 | Psoriasis | Adalimumab | Final dose: wk5 | Normal pregnancy/delivery: low birth weight |
| Andrulonis et al., 20122 | 22 | Recalcitrant pustular psoriasis and psoriatic arthritis | Ustekinumab | Throughout pregnancy | Normal pregnancy/normal delivery |
| Fotiadou et al., 20129 | 35 | Psoriasis | Ustekinumab | Final dose: 4wks preconception | Spontaneous abortion |
| Sheeran et al., 20144 | 34 | Recalcitrant psoriasis | Ustekinumab | Final dose: wk4 | Normal pregnancy/normal delivery |
| Sheeran et al., 20144 | 21 | Psoriasis | Ustekinumab | Final dose: wk2 | Pregnancy: outbreak of psoriasis in wk 34/normal delivery |
| Offiah et al., 20148 | - | Psoriasis and psoriatic arthritis | Infliximab | Throughout pregnancy | Normal pregnancy/delivery: collodion baby |
| Rocha et al., 20153 | 25 | Severe psoriasis | Ustekinumab | Final dose: 18d preconception | Normal pregnancy/normal delivery |
| Alsenaid et al., 20161 | 24 | Psoriasis/impetigo herpetiformis | Ustekinumab | Final dose: wk26 | Normal pregnancy/normal delivery |
Many reports have been published on pregnant patients with psoriatic arthritis, rheumatoid arthritis, or Crohn disease treated with biologic agents, but no previous case series or reviews have been published on patients with psoriasis treated with biologic therapy. Extrapolation of the results obtained in previous studies to patients with psoriasis would not appear wholly appropriate, as the characteristics, comorbidities, and idiosyncrasies of the diseases are different.
In 2009, a review was published of cases of congenital abnormalities reported to the FDA in children born to mothers who had received infliximab, etanercept, or adalimumab during pregnancy, independently of the underlying disease.10 Among the 120000 reported adverse reactions to these 3 drugs, the authors detected 41 cases of children born with congenital abnormalities. The main limitation of that study was that it did not enable incidences to be calculated, as the numerator (number of reported cases) was known but not the denominator (total population of pregnant women exposed to the drugs).
The results obtained in this review indicate a probable low risk of complications in women exposed to biologic agents during pregnancy, making the study somewhat reassuring for women accidentally exposed to these drugs. The decision to use this type of therapy during pregnancy must be made after appropriate evaluation of each case and determination of the risk-benefit ratio, that is, the balance between the importance of maintaining adequate control of the disease and the potential risk of fetal harm.
FundingBIOBADADERM is funded by the Spanish Academy of Dermatology and Venereology, by the Spanish Agency for Medicines and Healthcare Products, and by the pharmaceutical industry (Abbott, Merck-Schering Plough, and Pfizer-Wyeth). The participating companies each provide similar financial support and do not participate in the analysis or interpretation of results.
Conflicts of InterestA. Nuño-González has given lectures for Janssen Cilag, Roche, and IFC Cantabria.
F. Vanaclocha has given lectures for Abbott, Pfizer, MSD, and Janssen.
E. Daudén has undertaken the following activities: member of the Advisory Board, consultant, reception of grants, research support, participation in clinical trials, and receipt of fees for lectures given for the following pharmaceutical companies: Abbvie/Abbott, Amgen, Janssen Cilag, Leo Pharma, Novartis, Pfizer, MSD-Schering-Plough, Celgene, and Lilly.
M. Alsina has acted as a consultant for Pfizer, Abbvie, Jannsen, and MSD.
B. Pérez-Zafrilla has given lectures for Pfizer-Wyeth.
I. Belinchón has acted as a consultant for Pfizer-Wyeth; Janssen Pharmaceuticals Inc., MSD, Almirall SA, and Leo-Pharma, and has given lectures for AbbVie, Pfizer-Wyeth, Janssen Pharmaceuticals Inc., and MSD.
J. Sánchez-Carazo has acted as a consultant for AbbVie Laboratories, Janssen Pharmaceuticals Inc., MSD, and Pfizer-Wyeth.
The remaining authors declare that they have no conflicts of interest.
To all the participants collaborating in the BIOBADADERM register.
Gregorio Carretero, Carlos Ferrandiz, José Manuel Carrascosa, Raquel Rivera, Francisco José Gómez García, Pablo de la Cueva, Enrique Herrera, José Luis López Estebaranz, Mercè Alsina, José Luis Sánchez Carazo, and Marta Ferrán.
Please cite this article as: Echeverría-García B, Nuño-González A, Dauden E, Vanaclocha F, Torrado R, Belinchón I, et al. Serie de casos de pacientes psoriásicas expuestas a terapia biológica durante el embarazo. Registro BIOBADADERM y revisión de la literatura. Actas Dermosifiliogr. 2017;108:168–170.