In 1997, Birt, Hogg, and Dubé described multiple firm papules on the scalp, forehead, face, and neck in 15 persons belonging to a family of 70 individuals spanning 3 generations.1 The skin lesions were classified as 3 benign variants of hair follicle hamartomas: fibrofolliculomas, trichodiscomas, and acrochordons. This triad was later designated as the Birt-Hogg-Dubé syndrome (BHDS) (Online Mendelian Inheritance in Man catalog number, #135150).
A number of cases of BHDS have been identified since the original description was published, and significant associations have been reported, particularly with multiple lung cysts2,3 and renal cancer.2–4 A study in 2002 found that patients with BHDS have a 9.3-fold risk of developing renal tumors and a 32.3-fold risk of spontaneous pneumothorax.2
Case descriptionA 28-year-old man presented to our department with a 1-year history of skin lesions on the nose. The patient's past medical history was unremarkable, except for 2 spontaneous pneumothoraces at the age of 19 and 23 years. The patient also mentioned that his mother underwent right nephrectomy for a renal tumor at the age of 38 years.
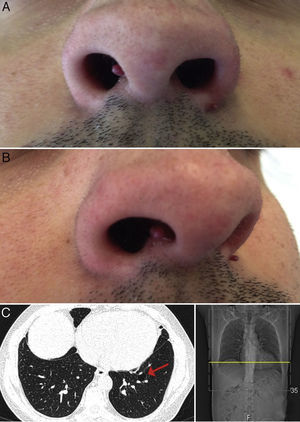
Physical examination revealed 2 wine-colored, pedunculated papules, 1 measuring 3mm on the right side of the columella, the other measuring 2mm on the left alar rim (Fig. 1A and B). No other significant alterations were observed on examination.
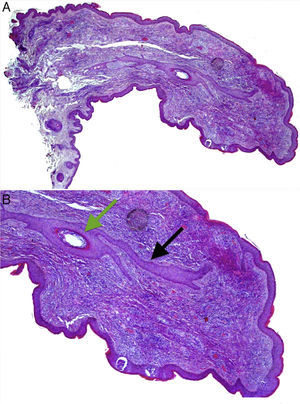
Both lesions were excised. Histological examination of the papule from the left alar rim was compatible with a vascular hamartoma. The lesion from the columella showed features compatible with a fibrofolliculoma (Fig. 2A and B).
(A) Histopathology of the papule located on the columella. Hematoxylin and eosin, original magnification 20×. (B) A central hair follicle (green arrow) with anastomosing bands of follicular epithelium extending from the central hair follicle into the adjacent stroma (black arrows), compatible with a fibrofolliculoma. Hematoxylin and eosin, original magnification 100×.
Sequencing of the coding exons (exons 4–14) and the intron-exon boundaries of the FLCN gene revealed a heterozygous FLCN:c.50G>C missense variant (p.Arg17Pro) in exon 4, both in the patient and in his mother. Numerous small cysts were observed in the basal regions of both lungs on computed tomography (CT) of the chest (Fig. 1C).
Applying the diagnostic criteria of the European BHD Consortium (Table 1),5 we made a diagnosis of BHDS based on 1 major criterion (an FLCN germline mutation) and 2 minor criteria (multiple lung cysts and a first-degree relative with BHDS).
Diagnostic criteria of the European BHD Consortium.
| Major | At least 5 fibrofolliculomas or trichodiscomas with onset in adult life and with at least 1 confirmed histopathologically |
| A pathogenic FLCN germline mutation | |
| Minor | Multiple lung cysts: bilateral basal cysts with no other apparent cause, with or without spontaneous pneumothorax |
| Multifocal or bilateral renal cancer of early onset (>50 years), or renal cancer of mixed chromophobe and oncoytic histology | |
| A first-degree relative with Birt-Hogg-Dubé syndrome | |
Diagnosis of Birt-Hogg-Dubé syndrome requires the presence of 1 major or 2 minor criteria.
Currently, the patient remains under regular follow-up because of the increased risk of developing renal cancer. Additionally, the patient's first-degree relatives have been referred for gene analysis.
BHDS is an inherited autosomal dominant disorder caused by germline mutations of the folliculin (FLCN) gene located on chromosome 17(17p11.2).5 BHDS-associated renal tumors display inactivation of the wild-type FLCN allele (for example, loss of heterozygosity, mutation, methylation), confirming that FLCN is a tumor suppressor gene that fits the classic 2-hit model.6,7 This gene encodes folliculin, a 579 amino acid protein expressed in a variety of tissues including stromal cells, the distal nephron, and type I pneumocytes, as well in the skin and skin appendages.8 This widespread expression of folliculin may explain the multisystem involvement of BHDS. Despite some recent research implicating possible interference with the energy-sensing mammalian target of rapamycin (mTOR) pathway in BHDS, the precise role of folliculin remains unknown. Most of the reported pathogenic FLCN mutations are of frameshift or nonsense types, which result in protein truncation.9 In contrast, the mutation identified in our patient, the heterozygous FLCN:c.50G>C, is a missense variant in exon 4 and has not previously been described in any patient or control.
In addition to identification of this novel mutation, our case is of particular interest because of the pedunculated clinical appearance of the fibrofolliculoma. Most cases of fibrofolliculoma and trichodiscoma present as multiple, dome-shaped, skin-colored or whitish papules on the face and neck. As observed in the majority of patients with BHDS, the skin lesions in our patient arose after the age of 20 years; however, it is estimated that 25% of all FLCN-mutation carriers older than 20 years will never develop skin lesions.10
As with the skin lesions, the involvement of internal organs in BHDS tends to develop after the second decade of life. The reported age range of renal cancer is of 25–75 years,5 and that of spontaneous pneumothoraces is of 22–71 years.3 Accordingly, the recommended screening of asymptomatic FLCN-mutation carriers should start at 20 years of age. There is no clear indication for routine CT-scanning of the lungs, and preventive measures are aimed largely at the early recognition and treatment of renal cancer. Annual renal MRI scans appear to be the best available screening method, with a high sensitivity and an absence of ionizing radiation. Whenever a pathogenic mutation is detected, gene analysis aimed at identifying and counseling asymptomatic family members should be offered.5
Our case underlines the importance of considering BHDS in the differential diagnosis of facial papules, even if inconspicuous or of atypical morphology. This is especially important if a positive personal or family history of pneumothorax or renal tumors is discovered. Finally, an early diagnosis of BHDS should be followed up with appropriate screening for renal cancer, not only for affected patients but also for their relatives who carry the FLCN mutation, improving their outcomes.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interestThe authors declare no conflict of interest.
We would like to thank Dr. J. Pardal, MD from the Department of Pathological Anatomy, Centro Hospitalar São João EPE in Oporto, Portugal, for providing the histologic images.