Imiquimod is an excellent option for patients with actinic keratosis, although its use may be limited by the long course of treatment required (4 weeks) and the likelihood of local skin reactions. The objectives of the present study were to demonstrate the effectiveness of a 12-day course of imiquimod 5% for the treatment of actinic keratosis and to examine the association between treatment effectiveness and severity of local reactions.
Patients and methodsWe included patients with at least 8 actinic keratoses treated with imiquimod 5% cream for 12 consecutive days. Local reactions were classified as mild, moderate, or severe. The statistical analysis of the association between local reactions and clinical response was based on the Pearson χ2 test and the Spearman rank correlation test.
ResultsSixty-five patients completed the study. Complete response was recorded in 52.3% and partial response in 75.4%. We found a statistically significant association between severity of the local reaction and response to treatment in both the Pearson χ2 test and the Spearman rank correlation test.
ConclusionsA 12-day course of imiquimod 5% proved effective for the treatment of actinic keratosis. Severity of local reactions during treatment was correlated with clinical response.
El imiquimod es un excelente tratamiento para las queratosis actínicas, sin embargo, su pauta prolongada de 4 semanas y la reacción local que produce pueden limitar su utilización. Los objetivos del estudio son demostrar la eficacia del imiquimod al 5% para las queratosis actínicas aplicado en una pauta continuada de 12 días y correlacionar el grado de reacción local con la eficacia.
Pacientes y métodosSe incluyeron pacientes con al menos 8 queratosis actínicas que se trataron con imiquimod en crema al 5% durante 12 días seguidos. Se evaluó la reacción local como leve, moderada o intensa. El estudio estadístico de la correlación entre la reacción local y la respuesta clínica se realizó con la prueba χ2 de Pearson y el test de correlación rho de Spearman.
ResultadosUn total de 65 pacientes completaron el estudio. Se obtuvo un 52,3% de respuestas completas y un 75,4% de respuestas parciales. Encontramos una asociación estadísticamente significativa entre el grado de reacción local y la respuesta al tratamiento tanto en la prueba χ2 de Pearson como en el test de correlación rho de Spearman.
ConclusionesLa pauta continuada de imiquimod al 5% aplicado durante 12 días es eficaz para el tratamiento de las queratosis actínicas. El grado de reacción local durante el tratamiento se correlaciona con la respuesta clínica.
Actinic keratosis (AK) is an increasingly interesting disease for dermatologists. It is defined as an intraepidermal neoplasm found on sun-damaged skin and characterized histologically by atypia of keratinocytes.1 Cryotherapy is the treatment used by most dermatologists in the case of solitary lesions. In patients with multiple AKs or patients with field cancerization, the approach used is the so-called field therapy. which can eliminate both clinically visible lesions and subclinical lesions corresponding to dysplasia of keratinocytes.2,3 Imiquimod is a drug used for the treatment of field cancerization and of which our experience and knowledge are increasing. The most common concentration is 5%, and the most common treatment schedule is 3 times weekly for 4 weeks. Its complex antitumor mechanism of action synergistically combines release of proinflammatory cytokines, antiangiogenic activity, and stimulation of apoptosis.4 The main adverse effect of treatment with imiquimod is a local skin reaction that manifests as erythema, pruritus, erosion, ulceration, crusting, and exudation in different degrees. The reaction first appears a few days after initiation of treatment, reaches maximum intensity at 4 weeks, and resolves approximately 2 weeks after completion of treatment. Severity seems to be dose-dependent5 and, taking into account its mechanism of action, could be associated with an improved response. Systemic adverse effects may also be observed during treatment with imiquimod owing to the absorption of the product and consequent release of interleukins and, in particular, interferon. These systemic findings involve flu-like symptoms, headache, nausea, myalgia, and arthralgia,5 and although they are well recognized and associated with treatment, they seem to be uncommon, with frequencies of 0.46% to 6% depending on the series.6–8 All the adverse effects, both local and systemic, can affect prescription of and adherence to imiquimod.
The present study was designed based on the hypothesis that intensification of the imiquimod regimen should not affect efficacy if an appropriate local reaction develops.
The main objective of the study was to demonstrate that daily application of imiquimod 5% cream for 12 consecutive days is an effective treatment for AK. The secondary objective was to study local and systemic adverse effects and establish a correlation between the degree of local reaction and the response to treatment.
Material and MethodsWe designed an uncontrolled clinical trial including patients with AK who were to receive treatment with imiquimod 5% cream. The inclusion criteria were an approximately 25-cm2 area on the face or scalp with at least 8 nonhypertrophic AKs. Patients were included in the study from July 2015 to October 2016.
The perioral and periocular areas were excluded as treatment areas. We also excluded patients who had received cryotherapy in the respective area during the 3 months before inclusion and patients who had received field therapy in the same area (imiquimod, photodynamic therapy, ingenol mebutate, 5-fluorouracil, or diclofenac) during the previous 6 months. We also excluded patients who had received immunosuppressive therapy and patients with hereditary diseases that predisposed to skin cancer (Gorlin syndrome, xeroderma pigmentosum).
The study was approved by the Ethics Committee of Instituto Valenciano de Oncología, Valencia, Spain. All patients gave their written informed consent to participate in the study.
At the initial visit, the area of the skin selected for treatment was photographed, and the AKs were counted and identified in the photograph. Patients were prescribed imiquimod 5% cream (Imunocare, Laboratorios IFC) and were instructed to apply 1 sachet of the product over the treatment area before bedtime for 12 consecutive days.
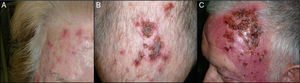
The end-of-treatment visit was attended 1-5 days after finishing the 12-day course. The patient was asked about the total number of sachets applied. At this visit, we evaluated the local skin reaction, which was classified as absent, mild, moderate, or intense (Fig. 1). Mild local reaction was defined as the appearance of discrete erythema and crusting limited to the AK lesions. Moderate local reaction was defined as the presence of erythema, edema, ulceration, and crusting in at least 50% of the treatment area. Intense local reaction was defined as the presence of erythema, edema, ulceration, crusting, and exudate that covered almost the complete treatment area and even affected skin outside the treatment area. Furthermore, at this visit, patients were asked if they had developed any treatment-associated systemic symptoms such as low-grade fever, asthenia, headache, nausea, myalgia, or arthralgia, in which case a systemic reaction was considered to be present. The end-of-study visit was held approximately 1 month after the end-of-treatment visit. Efficacy of treatment was classed as complete clinical response (CCR) or a partial clinical response (PCR). The response was defined as the percentage of AKs that had resolved at the visit and calculated as the number of AKs counted after treatment divided by those counted before treatment. CCR was defined as total absence of AKs or lesions clinically suspected of being AKs and confirmed through visual evaluation and touch; PCR was defined as a reduction of more than 75% in the initial AKs evaluated through visual exploration and touch.9
The association between the local reaction and complete response to treatment was evaluated using contingency tables; differences in distribution were compared using the Pearson χ2 test. Furthermore, the percentage of AKs resolved was associated with the degree of local reaction using the Spearman rank correlation coefficient. Statistical significance was set at P<.05. The analysis was performed using SPSS version 15.0 (SPSS Inc).
ResultsThe study population comprised 71 patients. Patient characteristics are presented in Table 1. Mean age was 77.8 years, and most patients were men (65 [91.5%] vs 6 women [8.5%]). The median number of AKs was 12 (8-30), and the most frequent site was the scalp (29 cases, 40.8%).
A total of 65 patients attended the end-of-treatment and end-of-study visits.
At the end-of-treatment visit, 59 of the 65 patients had applied all 12 sachets, and the remaining 6 patients had suspended treatment because of adverse effects. One patient applied 11 sachets, 2 patients 10, 1 patient 8, and 2 patients only 7. Table 2 shows the results for the local reaction. All of the patients developed some degree of local reaction, which was intense in 58.5% of cases. The 6 patients who used fewer than 12 sachets had an intense local reaction.
Thirteen patients (20%) reported systemic symptoms, most of which were general malaise, low-grade fever, and flu-like symptoms. Only 3 of these patients interrupted treatment before 12 days. Two patients used 7 sachets and 1 used imiquimod for 8 consecutive days. The local reaction was intense in 11 cases and moderate in 2.
As for the efficacy of the 12-day course of imiquimod 5%, we obtained a CCR in 34 patients (52.3%) and a PCR in 49 (75.4%).
The results for the association between the local reaction and complete response to treatment are shown in Table 3. In the case of the 38 patients who developed an intense local reaction, 27 (71.1%) achieved a CCR; however, most of the patients who experienced a mild or moderate local reaction did not have a CCR. These differences were statistically significant. The Spearman rank correlation coefficient yielded a rho of 0.557 (P>.0001). Therefore, a moderate positive correlation was observed between the local reaction and response to treatment; in other words, the more intense the local reaction, the better the response to treatment. Six of the 38 patients who had an intense local reaction had discontinued treatment before the 12 days. Of these, 5 (83%) had a CCR. Of the other 32 cases with an intense local reaction, 22 had a CCR (69%).
DiscussionImiquimod 5% has been used to treat AK for almost 20 years. While it is well known to produce a local reaction, many studies and broad experience confirm its safety and efficacy. However, both the local reaction and the treatment regimen (usually 4 weeks) may affect how the drug is prescribed. Our results show that a 12-day schedule of imiquimod 5% cream is an effective treatment for AK. We found that this continuous regimen cured all of the AKs in 52.3% of cases, and that more than 75% of the lesions resolved in 49 patients (75.4%). Our findings are similar to those of the meta-analysis by Hadley et al.,10 which included 1293 patients with AK and revealed a CCR of 50% and a PCR of 65%. Furthermore, when these results are compared with those for other topical AK treatments—CCR of 42.2% with ingenol mebutate11 and 39.6% with diclofenac12—a continuous regimen with imiquimod seems very effective. Our results are particularly interesting for 2 reasons. First, the pivotal studies with imiquimod used to treat AK included in the meta-analyses published to date report regimens of 12-16 weeks,10,13 which is significantly longer than that reported in the present study. In various studies where imiquimod is applied 3 times weekly for 4 weeks (ie, the regimen recommended in the summary of product characteristics), the CCR reported ranges from 26.8% to 40.5%,8,9,14,15 which is lower than in the present study. Furthermore, the patients included in the present study had a higher number of AKs than that reported in most studies on imiquimod6,14–17 and on other topical AK treatments with less ambitious inclusion criteria, ie, patients with only 5 AKs.11,18 Therefore, this continued regimen with imiquimod could be recommended, first, because of its apparent efficacy and, second, because it considerably reduces the time taken for application and resolution of the local reaction, which can last up to 2 months from initiation of treatment with the traditional regimen.
Many published studies suggest that the degree of local reaction produced by imiquimod is dose-dependent and that it seems to be associated with a better response.13,19–21 However, these suggestions are based mainly on observations and findings in the discussion section and were not included among the objectives of the studies. In this context, and based on our experience, we developed the second objective in our study, where we demonstrated, with statistically significant differences (Pearson χ2 contingency table and Spearman rank correlation coefficient), that the degree of local reaction during treatment with imiquimod can predict response. Patients who experience a more intense reaction, that is, those who normally attend the clinic, are precisely the group that best respond. This observation may help us to explain to them during their visit that the treatment is successful precisely because of the reaction.
Furthermore, we found that many patients reported systemic symptoms (20%). According to our literature search, the percentage of patients with systemic symptoms was lower than 6%, especially for headache. These adverse effects are probably due to systemic absorption of the drugs and release of interferon. Once again, the effects seem to be dose-dependent. In patients who experienced a more intense local reaction, continued application of treatment on the inflamed and eroded skin leads to better absorption and a greater probability of systemic symptoms.7 Specifically, 11 of the 13 patients with systemic symptoms in the present study had also developed an intense local reaction, thus favoring the hypothesis. Therefore, it seems sensible to propose imiquimod for treatment of AK until a local reaction that guarantees the efficacy of treatment appears, albeit without the need to prolong this reaction in order to prevent systemic symptoms. In this sense, Dirschka et al.22 propose personalizing treatment with imiquimod 3.75% depending on the local reaction. Furthermore, the authors of a study in Australia achieved very good results with a regimen of imiquimod under occlusion for only 1 week in patients with nonmelanoma skin cancer.23 Moreover, according to our results—discontinuation in 9% of patients, intense local reaction in 58%, and systemic symptoms in 20%—we can conclude that although the efficacy of this new regimen seems superior to the conventional regimen, it may be at the expense of lower tolerance. Thus, it is noteworthy that the patients with an intense local reaction include 6 who discontinued treatment before 12 days and that 5 of these patients had a CCR, that is, 83% compared with a CCR of 69% among patients with an intense local reaction. Therefore, it could prove interesting to use imiquimod until a grade of local reaction that guarantees efficacy is reached and that can be reached within a specific timeframe. We insist that it is necessary to strike a balance between the local reaction, tolerance, and efficacy in order to tailor treatment.
Our study is subject to 2 limitations. First, we did not calculate the sample size with the aim of reaching the study objective (ie, determination of the efficacy of the new regimen). Second, there was no control group with the conventional regimen.
In conclusion, we present a continuous regimen with imiquimod 5% for the treatment of AK. This regimen produces an adequate local reaction, which is the same as that obtained with the conventional regimen, thus ensuring the mechanism of action of the drug and, therefore, its efficacy.
Conflicts of InterestC. Serra-Guillén gives paid talks for Laboratorios IFC. The remaining authors declare that they have no conflicts of interest.
Please cite this article as: Serra-Guillén C, Nagore E, Llombart B, Sanmartín O, Requena C, Calomarde L, et al. Tratamiento con imiquimod al 5% durante 12 días para las queratosis actínicas: estudio de la eficacia y la reacción local. Actas Dermosifiliogr. 2018;109:248–253.