Zika virus infection should be suspected in travelers or immigrants with the signs or symptoms of a viral infection (rash, fever, joint pains, conjunctivitis, headache, etc.) and a compatible epidemiological history. Although cutaneous manifestations are among the most common clinical signs of Zika, they are not specific and very few images are available. We present 3 patients (2 travelers and 1 immigrant) in whom a rash was the presenting manifestation of Zika virus infection. Prompt diagnosis optimizes outcomes in these patients, improves the management of severe disease, and minimizes the risk of local transmission by Aedes albopictus, now a potential local vector for the virus due to its presence in areas along Spain's Mediterranean coast.
La infección por el virus Zika debe sospecharse en viajeros o inmigrantes con clínica de viriasis (exantema, fiebre, artralgias, artritis, conjuntivitis, cefalea, etc.) y una historia epidemiológica compatible. Aunque las manifestaciones cutáneas se encuentran entre las más frecuentes no son específicas y su iconografía es escasa. Presentamos 3 casos, 2 viajeros y un inmigrante que comienzan con un exantema por virus Zika. Alcanzar el diagnóstico de forma rápida optimiza el manejo de estos pacientes, mejora el control de los casos graves y permite minimizar una posible transmisión autóctona dado el riesgo que supone la presencia del Aedes albopictus como potencial vector transmisor de esta enfermedad en el litoral mediterráneo español.
The global impact of Zika virus infection is undeniable.1 In Spain, a high level of clinical suspicion is warranted given the large numbers of persons of Latin American origin and the country's thriving tourism industry. High-risk areas of Spain include the Mediterranean coast and also Aragon and the Basque Country, given the presence of Aedes albopictus, a potential vector of the virus.2 Although skin manifestations are very common in Zika virus infections, few images are available in the literature.
We describe the cases of 3 patients who came to our clinic with skin lesions after returning to Spain from Latin America. All had received multiple mosquito bites and presented with fever and a nonspecific erythematous maculopapular rash. Zika virus infection was confirmed upon polymerase chain reaction (PCR) detection of viral nucleic acids in serum and urine. All 3 patients progressed favorably.
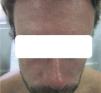
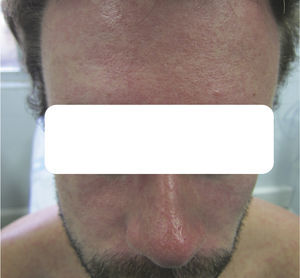
Case DescriptionsCase 1The patient was a 25-year-old Spanish man who had returned to Spain from Martinique 7 days earlier. He reported no symptoms during his stay except for numerous arthropod bites, but attended our outpatient clinic with pruritic skin lesions on the upper half of his body that had developed 24hours earlier together with fever and asthenia. The patient's general condition was good, and his temperature was 38.3°C. He had a confluent erythematous maculopapular rash that predominantly affected the face (Fig. 1) and trunk (Fig. 2). There were scattered lesions on the lower limbs. The results of a complete blood count (CBC) and tests of liver function, coagulation, and acute phase reactants (C-reactive protein and procalcitonin) were normal. We prescribed treatment for symptoms and ordered tests for cytomegalovirus (CMV), Epstein-Barr virus (EBV), hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV), dengue virus, chikungunya virus, Zika virus, Rickettsia conorii, and syphilis. The results of all tests were negative, except for PCR for Zika virus, which was detected in both serum and urine samples. The patient recovered fully within a week.
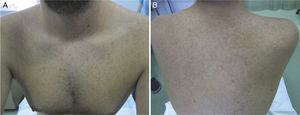
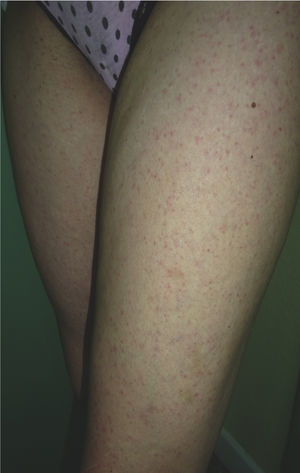
The patient was a 31-year-old Spanish woman who had returned 2 days earlier from Santo Domingo in the Dominican Republic. She reported no clinically relevant events except for arthropod bites, but consulted us for a centrifugally distributed generalized cutaneous rash that had appeared abruptly 24 hours earlier together with self-limiting diarrhea and a mild frontal headache. The patient's general condition was good, and her temperature was 37.7°C. An erythematous maculopapular rash was observed on the cheeks, the anterior and posterior aspects of the trunk, the limbs, and the palms (Fig. 3). Laboratory results (CBC, ionogram, and tests of liver function, coagulation, and acute phase reactants) were normal. We prescribed treatment for symptoms. The rash had resolved by a follow-up examination 24hours later. The results of microbiological tests for CMV, EBV, HAV, HBV, HCV, HIV, rubella and measles viruses, dengue virus, chikungunya virus, Zika virus, and syphilis were negative, except for PCR detection of Zika virus in urine. The patient recovered fully within 72hours of onset.
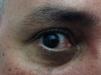
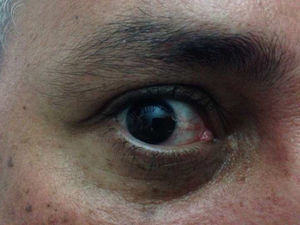
Case 3The patient was a 46-year-old Venezuelan man who lives in Spain. He reported no clinically relevant events during his most recent visit to Venezuela, except for numerous arthropod bites. Two days after returning to Spain he consulted us for skin lesions in the upper half of his body that had appeared several hours earlier. The rash was accompanied by fever, general malaise, pain on swallowing, myalgia, and small joint pain. The patient's general condition was good, and his temperature was 39.5°C. He had mucosal dryness and bilateral conjunctival hyperemia (Fig. 4) together with a confluent erythematous maculopapular rash mainly on the face, trunk, and back and blanched when pressed with a glass slide. The results of the CBC and liver function and coagulation tests were normal: C-reactive protein, 37.0mg/dL; procalcitonin, 1.27ng/mL. Microbiology (for CMV, EBV, HAV, HBV, HCV, HIV, chikungunya virus, and syphilis) results were negative but showed markers of a previous dengue virus infection. The results of serology and blood PCR for Zika virus were negative. Urine PCR produced a positive result for Zika virus. The patient improved after 24hours.
DiscussionZika virus is an arthropod-borne virus (arbovirus) (genus Flavivirus, family Flaviviridae) that was first identified in Uganda in 1947.3 Transmission occurs via coexisting urban and sylvatic cycles and involves mosquito vectors of the genus Aedes. Sexual, vertical, and blood-transfusion transmission have also been described.4,5
Zika virus was limited to specific regions of Africa and Asia until 2007. Since then, the virus has spread unrestrained throughout the world. The cases reported to date in Spain have been the result of infections acquired in other countries; congenital and sexual transmission has been described6,7 (HYPERLINK “http://www.msssi.gob.es/profesionales/saludPublica/zika/casosDiagnosticados/home.htm”). Zika virus infection can potentially have a substantial impact in Spain given the following factors: 1) Spain's thriving tourism industry; 2) the large number of Latin Americans resident here; 3) the presence along the Spanish Mediterranean coast of Aedes albopictus, which could theoretically mediate autochthonous transmission after biting an individual infected abroad; and 4) the possibility of sexual transmission.2 Prompt diagnosis would allow optimization of clinical management, improve the monitoring of severe cases, and minimize the risk of potential autochthonous transmission. Early detection in pregnant women is essential to detecting cases of congenital Zika virus infection.
Most patients (75%–80%) are asymptomatic.8 The remainder present with rash that is occasionally pruritic (90%), fever (65%), joint pain and inflammation (65%), nonpurulent conjunctivitis (55%), headache (45%), and less frequently digestive symptoms (10%).5 In some cases Zika virus infection can be associated with neurological signs.9 The most important thing to remember about this disease is its ability to cause fetal malformations and miscarriage.10
Although skin lesions are common in Zika infections, they are not often illustrated in the literature. The lesions are nonspecific and often difficult to distinguish from those caused by other arboviruses such as dengue and chikungunya viruses (Table 1). Furthermore, coinfections with other tropical viruses can complicate diagnosis.11 The rash caused by Zika virus consists of erythematous morbilliform or scarlatiniform macules and/or papules that appear on the trunk or face 3 to 5 days after onset of the febrile phase. In the cases we report the cutaneous manifestations resolved within 24hours. The differential diagnosis should include other infections such as rubella, parvovirus, measles, dengue and chikungunya viruses, leptospirosis, and rickettsiosis.11 Zika virus infection can be distinguished from dengue and chikungunya infections by certain clinical signs. Chikungunya virus causes characteristic polyarthralgia and joint pain and inflammation that can persist for months. Auricular chondritis, a highly characteristic symptom, is sometimes present. Dengue virus infections can be distinguished by the presence of intense myalgia and a purpuric rash predominantly affecting the lower limbs and dependent areas. Zika virus infection usually causes low-grade fever and few systemic manifestations. A definitive diagnosis is established by PCR detection of viral RNA in biological fluids during the acute phase of infection (in blood for the first 5 days and in urine during 10 to 15 days) and by the detection of immunoglobulin M antibodies by enzyme-linked immunosorbent assay or immunofluorescent antibody assay during the convalescent phase.11 Given the possibility of cross-reactions with other flaviviruses, the results of these assays should be confirmed by virus neutralization testing. Furthermore, in patients who come from areas where other flaviviruses coexist with the Zika virus, serum neutralization assays are required for a specific diagnosis.12
Cutaneous Manifestations of Zika Virus Infection.
| Edema and erythema of the malar region of the face |
| Pruritic maculopapular rash with a follicular distribution |
| Trunk and limbs |
| Face |
| Palms and soles |
| Rash morphology |
| Morbilliform |
| Scarlatiniform |
| Conjunctival injection |
| Submandibular and cervical lymphadenitis |
| Mild hemorrhagic manifestations |
| Gingival bleeding |
| Petechiae and hyperemia on the hard palate |
| Post-rash dry skin and scaling |
Given the current lack of any specific treatment or prophylaxis for Zika virus infection, symptomatic treatment and vigilance to detect complications are necessary.5
In conclusion, early clinical suspicion of Zika virus infection can help minimize possible autochthonous transmission and optimize disease management. Zika virus infection should therefore be included in the differential diagnosis in any case of nonspecific febrile rash and a compatible epidemiological history.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Data confidentialityThe authors declare that they followed their hospital's regulations regarding the publication of patient information.
Right to privacy and informed consentThe authors obtained the informed consent of the patients referred to in this article. The signed forms are in the possession of the corresponding author.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
The authors thank Leire Sánchez Los Arcos, Fernando de Ory, and André Barbosa for their contributions to this study.
Please cite this article as: Cosano-Quero A, Velasco-Tirado V, Seco MPS, Manzanedo-Bueno L, Belhassen-García M. Virus Zika: manifestaciones cutáneas en 3 pacientes. Actas Dermosifiliogr. 2018;109:e13–e16.