Patients with atopic dermatitis (AD) often require systemic immunosuppressant treatment, but the use of these drugs is limited by their potential toxicity and/or an insufficient therapeutic response. Recently, Puya et al.1 published the case of a patient with severe AD successfully treated with ustekinumab.
We present a retrospective descriptive study of patients with severe AD treated with ustekinumab in our hospital, a reference hospital, between December 2009 and February 2011. There were 4 patients, all men aged between 23 and 29 years, who presented severe AD refractory to oral corticosteroids, phototherapy (Psoralen–UV-A or narrowband UV-B), and to at least 2 systemic drugs, including ciclosporin, azathioprine, methotrexate, and mycophenolate mofetil. All the patients had negative Mantoux and booster effect, negative serology for hepatitis B and C viruses and human immunodeficiency virus, and no history of other systemic diseases, serious infections, or tumors (Table 1). Ustekinumab was prescribed for off-label use, with approval by medical management, and the patients signed the corresponding informed consent.
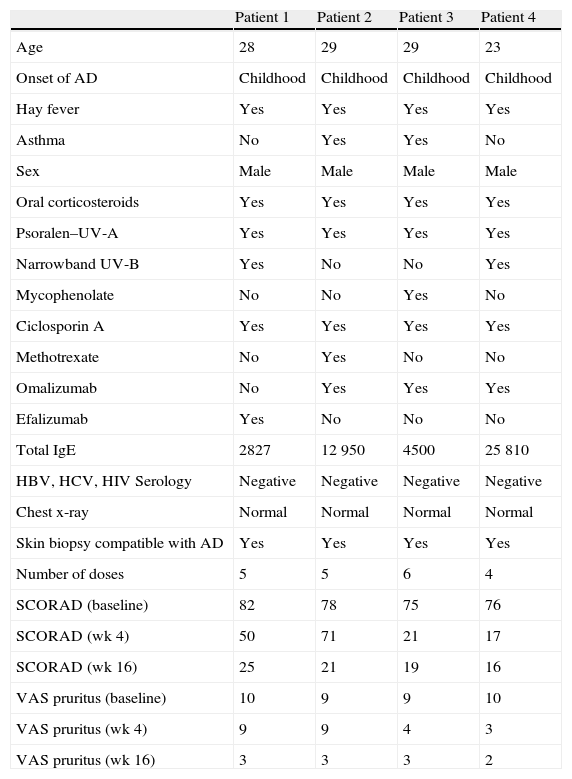
Summary of the Epidemiological, Clinical, and Therapeutic Characteristics of our Patients.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
| Age | 28 | 29 | 29 | 23 |
| Onset of AD | Childhood | Childhood | Childhood | Childhood |
| Hay fever | Yes | Yes | Yes | Yes |
| Asthma | No | Yes | Yes | No |
| Sex | Male | Male | Male | Male |
| Oral corticosteroids | Yes | Yes | Yes | Yes |
| Psoralen–UV-A | Yes | Yes | Yes | Yes |
| Narrowband UV-B | Yes | No | No | Yes |
| Mycophenolate | No | No | Yes | No |
| Ciclosporin A | Yes | Yes | Yes | Yes |
| Methotrexate | No | Yes | No | No |
| Omalizumab | No | Yes | Yes | Yes |
| Efalizumab | Yes | No | No | No |
| Total IgE | 2827 | 12 950 | 4500 | 25 810 |
| HBV, HCV, HIV Serology | Negative | Negative | Negative | Negative |
| Chest x-ray | Normal | Normal | Normal | Normal |
| Skin biopsy compatible with AD | Yes | Yes | Yes | Yes |
| Number of doses | 5 | 5 | 6 | 4 |
| SCORAD (baseline) | 82 | 78 | 75 | 76 |
| SCORAD (wk 4) | 50 | 71 | 21 | 17 |
| SCORAD (wk 16) | 25 | 21 | 19 | 16 |
| VAS pruritus (baseline) | 10 | 9 | 9 | 10 |
| VAS pruritus (wk 4) | 9 | 9 | 4 | 3 |
| VAS pruritus (wk 16) | 3 | 3 | 3 | 2 |
Abbreviations: AD, atopic dermatitis; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; Ig, immunoglobulin; SCORAD, Scoring Atopic Dermatitis; VAS, visual analogue scale (range, 1-10).
A visual analogue scale (VAS) for pruritus and the Scoring Atopic Dermatitis (SCORAD) index were the tools used to quantify the results, both at baseline and during weeks 4 and 16.
The 4 patients were aged between 23 and 29 years (Table 1). The number of injections of ustekinumab during the study period varied between 4 and 6. In all cases the patients were administered subcutaneous doses of 45mg of the drug, using the regimen usually employed in psoriasis: injections in weeks 0 and 4 and then every 12 weeks. No adverse effects, infections, or other complications were observed during the study period.
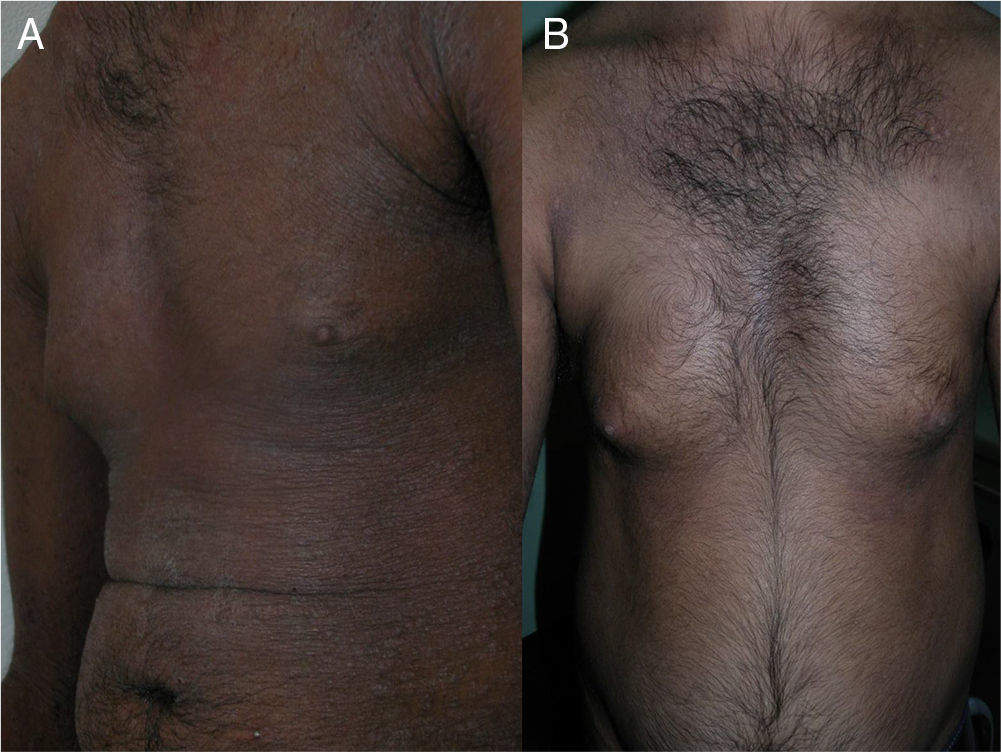
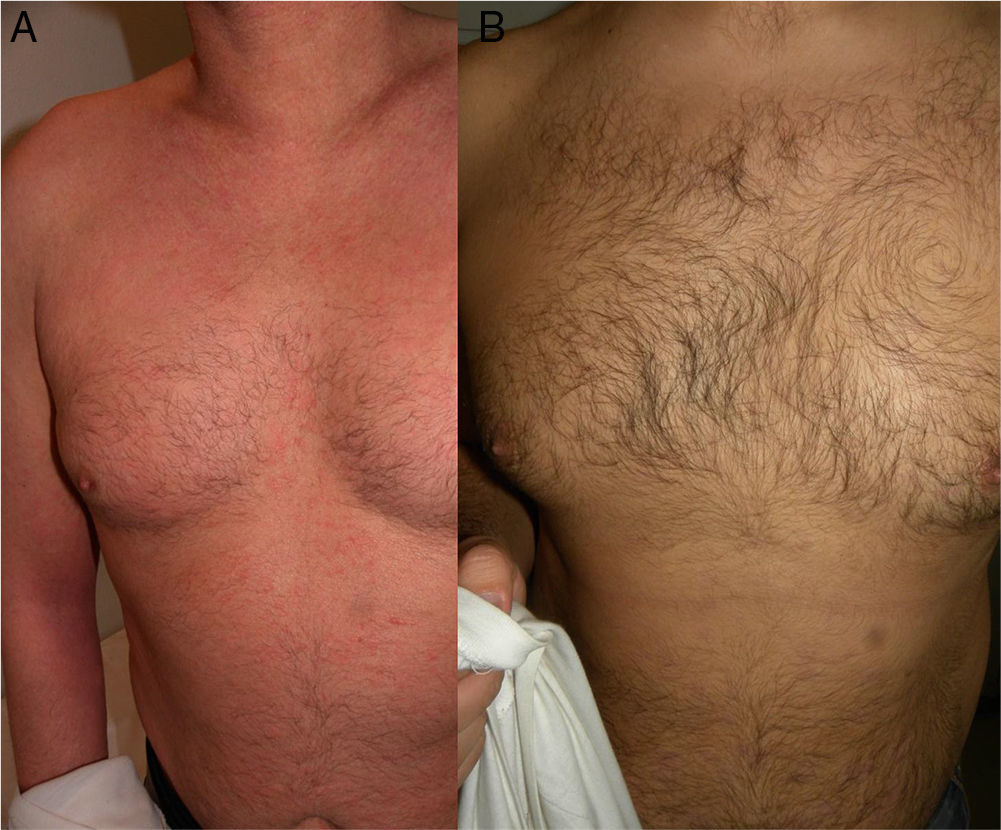
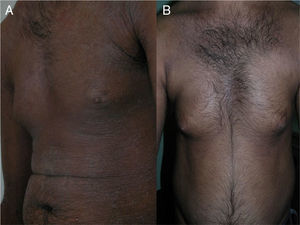
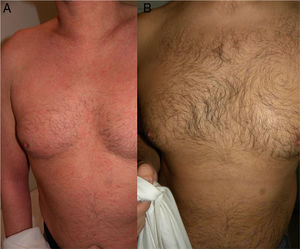
The skin lesions, the pruritus, and the patient-perceived quality of life improved after the second injection of ustekinumab (week 4) in 2 of the patients and after the third injection (week 16) in the other 2 (Figs. 1 and 2), as evidenced by the marked improvements in the scores of the VAS for pruritus and the SCORAD index (Table).
The mechanism of action of ustekinumab in AD is not fully understood.2,3 One possibility that has been suggested is an inhibition of the Th17 pathway4; this will prevent the process of presentation of epidermal autoantigens.3,5 The Th17 pathway and its associated cytokines, interleukin (IL)-17 and IL-22, also have proinflammatory effects on a wide variety of cells, including keratinocytes, macrophages, and endothelial cells, and this pathway is overexpressed in AD and other inflammatory disorders.6 In 2003, Toda et al. found elevated levels of IL-17 in acute AD lesions. Koga et al. demonstrated that the percentage of Th17 helper T cells in the peripheral blood was elevated in patients with AD in comparison with a group of healthy individuals, and that this was associated with the severity of AD. These data suggest the participation of Th17 cells in AD.
In our series we observed a good clinical response, with resolution of the episodes of generalized eczema and erythroderma and subjective improvements in the pruritus and patient-perceived quality of life, but a prospective study would be necessary to evaluate these findings.
We conclude that ustekinumab is a potentially useful treatment in patients with severe AD that is refractory to conventional therapy, although further studies are required to evaluate both its effectiveness and its long-term safety. A further controversial issue is the high cost of treatment, and a careful cost-efficacy assessment must therefore be performed.
Please cite this article as: Fernández-Antón Martínez M, Alfageme Roldán F, Ciudad Blanco C, Suárez Fernández R. Ustekinumab en el tratamiento de la dermatitis atópica severa. Nuestra experiencia en 4 pacientes. Informe preliminar. Actas Dermosifiliogr. 2014 105:312–313.