Molluscum contagiosum is one of the most common viral infections in childhood. It is a benign and usually self-limiting infection, but its treatment in children can be challenging, particularly when the patient presents multiple lesions or when lesions are symptomatic or highly visible. Several treatment options exist. Choice of treatment depends on the number and location of lesions, the prior experience of the treating physician, and the preferences of the child's parents or carers. This article provides an update on treatment options for molluscum contagiosum, with a particular focus on immunocompetent pediatric patients.
El molusco contagioso es una de las infecciones virales más frecuente en los niños. Aunque se trata de una infección de curso benigno y generalmente autolimitada, el tratamiento puede resultar complicado en la edad pediátrica cuando las lesiones son muy numerosas, están en áreas visibles, o producen molestias. Existen diversos tratamientos disponibles, cuya selección depende del número y localización de las lesiones, de la experiencia del médico que las trata, y de las preferencias de los padres o cuidadores. Este artículo proporciona una actualización sobre las diferentes terapias contra los moluscos contagiosos particularmente enfocadas a los pacientes pediátricos.
Molluscum contagiosum (MC) is caused by a DNA virus of the genus Molluscipoxvirus, family Poxviridae. Currently, this virus is categorized into 2 types (MCV-1 and MCV-2) and 4 distinct genotypes.1 Genotype 1 accounts for 98% of cases recorded in the United States, genotypes 2 and 3 are more prevalent in Europe and Australia and in patients with human immunodeficiency virus 1, and genotype 4 is rare.2 MC is one of the 50 most frequent diseases worldwide.3 In children its annual incidence ranges from 2% to 10%4 and its prevalence from 5.1% to 11.5%.5 However, these rates vary significantly depending on the population studied. MC can be transmitted by direct contact, fomites, and self-inoculation.1 The incubation period ranges from 14 days to 6 months. Unlike herpesvirus, MC does not persist as a latent infection. The review of the literature of an Australian survey of MC patients revealed that it mainly affects school-aged children who have visited a swimming pool.6 However, there is no documented evidence demonstrating that transmission can be effectively prevented by keeping children out of pools.7 Other variables such as direct contact, the presence of fomites, and living in tropical climates are also associated with higher rates of infection.6 Another study determined that individuals who share a bath sponge or towel with an infected patient have a 3-fold greater relative risk of infection than those who do not share these items.8 Certain preventive measures (eg, bathing children alone, avoiding shared use of sponges and towels, and covering MC lesions) may therefore be effective.
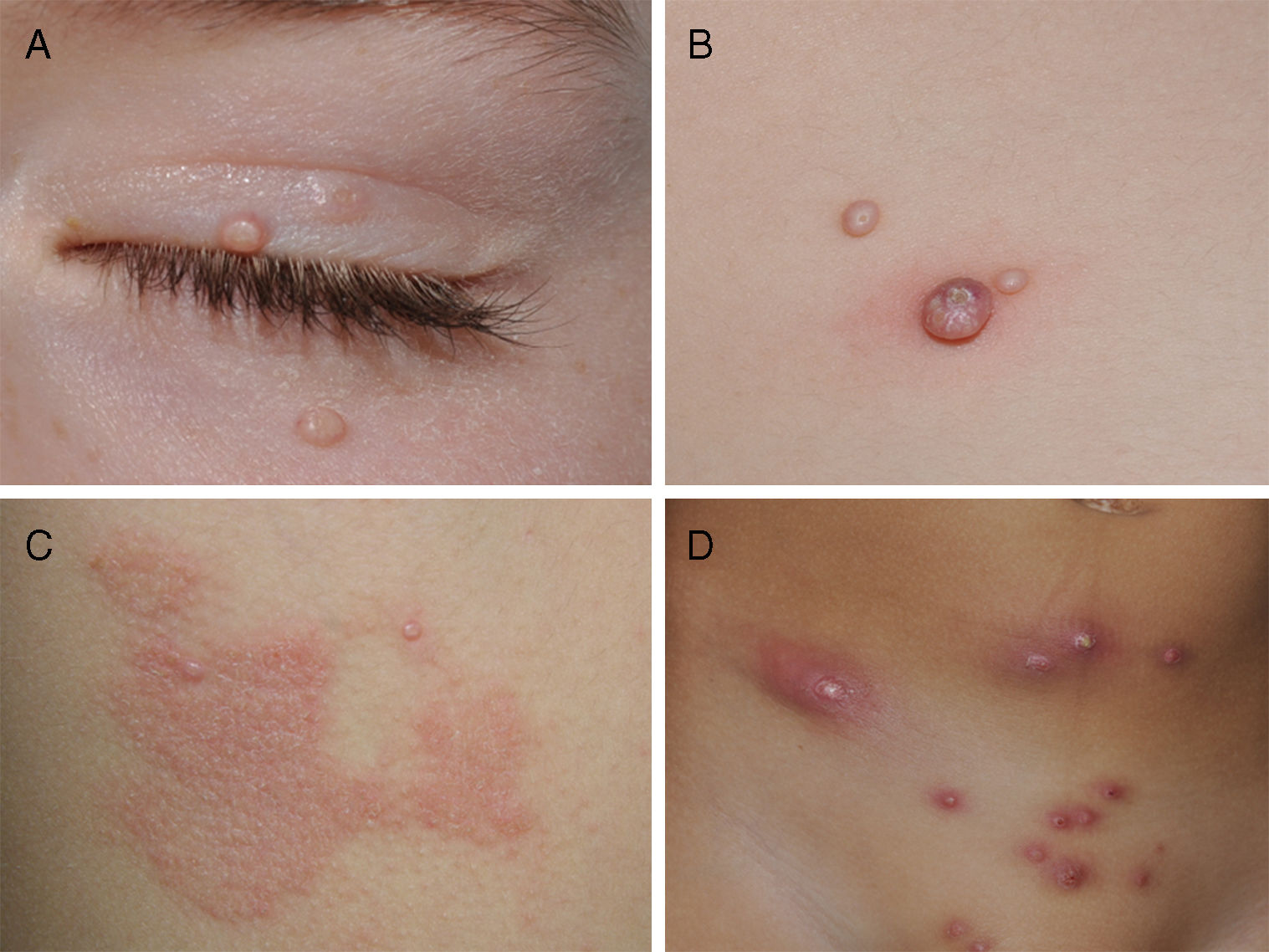
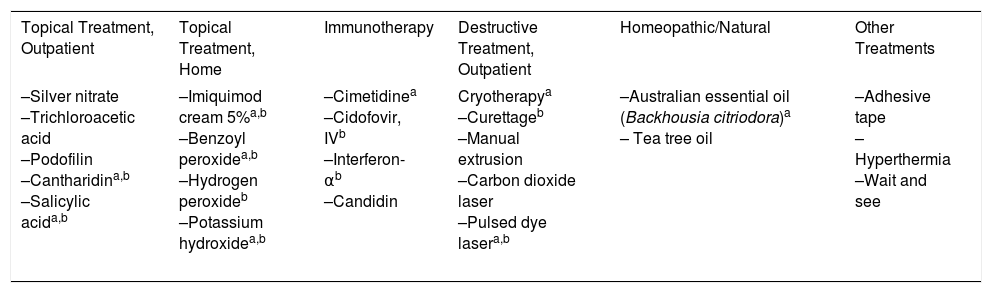
Clinically, MC is characterized by skin-colored papules and/or nodules with central umbilication. In some patients, these lesions may be surrounded by a halo of eczema, known as molluscum dermatitis.9 This is the result of a hypersensitivity reaction to the viral antigen2 and can evolve into an abscess or a less morphologically typical lesion (Fig. 1). While any area of the skin or mucous membranes can be infected, lesions on the soles, palms, and mucous membranes are rare.6 Children often develop associated atopic dermatitis (AD). In a retrospective medical chart review of 696 pediatric MC cases, 259 (37.2%) had a history of AD and 38.8% had molluscum dermatitis.9 In patients with underlying AD or other conditions associated with compromised immunity, lesions tend to be more numerous and longer lasting.2
Different clinical manifestations of molluscum contagiosum (MC). A, Pink papules on the eyelids with typical central umbilication. B, Sessile lesion of less typical morphology next to other lesions more characteristic of MC. C, Eczematiform reaction (molluscum dermatitis) surrounding MC lesions. D, Inflamed and abscessed lesions on the abdomen.
In immunocompetent patients, skin infections caused by MC are benign and self-limiting. There are multiple treatment options available, none of which is significantly more effective than the other.10 In selecting a treatment for pediatric patients, the priorities should be to avoid pain and minimize the risk of scarring. Furthermore, it is essential to reassure parents and inform them as to the expected course of the disease and treatment outcome. A survey of parents of children with MC found that they were mainly concerned about scarring, pruritus, the possibility of contagion, pain, and the effects of treatments.6 However, children's quality of life was not affected.
Types of Treatment for MCTreatment options for MC lesions are listed in Table 1. Those that have been used in pediatric patients are described below.
Treatment Options for Molluscum Contagiosum and Corresponding Degree of Evidence.
| Topical Treatment, Outpatient | Topical Treatment, Home | Immunotherapy | Destructive Treatment, Outpatient | Homeopathic/Natural | Other Treatments |
|---|---|---|---|---|---|
| –Silver nitrate –Trichloroacetic acid –Podofilin –Cantharidina,b –Salicylic acida,b | –Imiquimod cream 5%a,b –Benzoyl peroxidea,b –Hydrogen peroxideb –Potassium hydroxidea,b | –Cimetidinea –Cidofovir, IVb –Interferon-αb –Candidin | Cryotherapya –Curettageb –Manual extrusion –Carbon dioxide laser –Pulsed dye lasera,b | –Australian essential oil (Backhousia citriodora)a – Tea tree oil | –Adhesive tape –Hyperthermia –Wait and see |
Abbreviations: IV, intravenous.
Destructive methods are the most commonly used methods in routine practice and result in the destruction of keratinocytes infected by the MC virus. These simple and inexpensive procedures, when carried out by a suitably qualified health care professional, are very effective.2
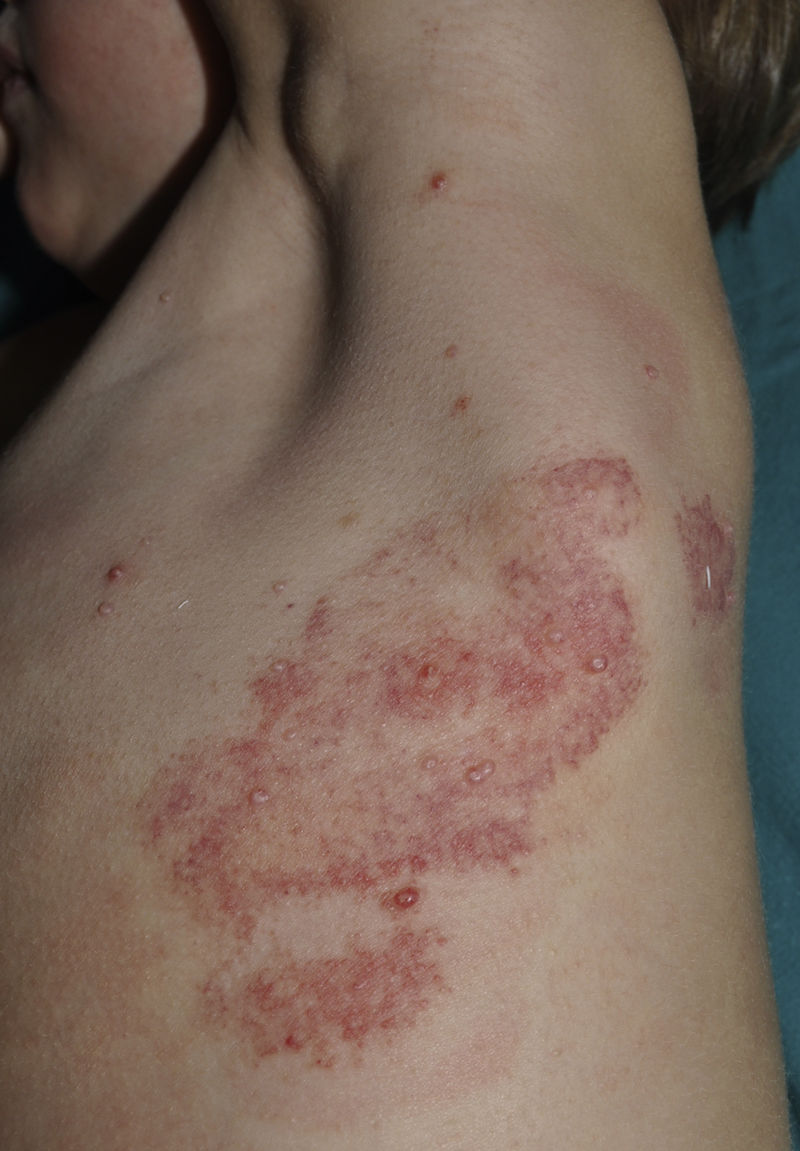
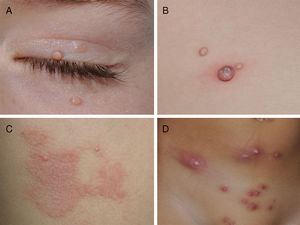
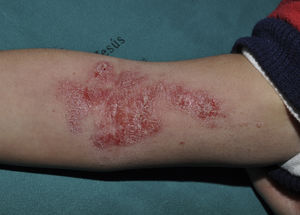
CurettageCurettage is a simple and relatively inexpensive procedure, with the added advantage that the tissue removed can be kept for histopathological analysis in case of diagnostic doubt.11 EMLA cream, a eutectic mixture of local anesthetics (2.5% lidocaine and 2.5% prilocaine), is frequently used in children to ameliorate the pain caused by the procedure, although its application on MC lesions can cause local, self-resolving purpuric reactions12,13 (Fig. 2). The risk of systemic toxicity should also be considered if EMLA is applied to a large area, particularly in infants less than 3 months old14 (Table 2). Curettage is probably one of the most effective methods. A retrospective clinical study of 1879 pediatric patients found that 70% were cured after a single treatment, 26% required 2 treatments, and only 4% required 3 treatments.15 Satisfaction was high (97% in children and parents). A randomized, controlled trial comparing the efficacy of curettage, cantharidin, salicylic acid with glycolic acid, and imiquimod found that curettage was the most effective therapy, resulting in complete resolution in 80.6% of patients with no recurrences after 6 months of follow-up.16 Disadvantages of curettage include the need for local anesthesia, potential pain and bleeding, and the risk of scarring.17
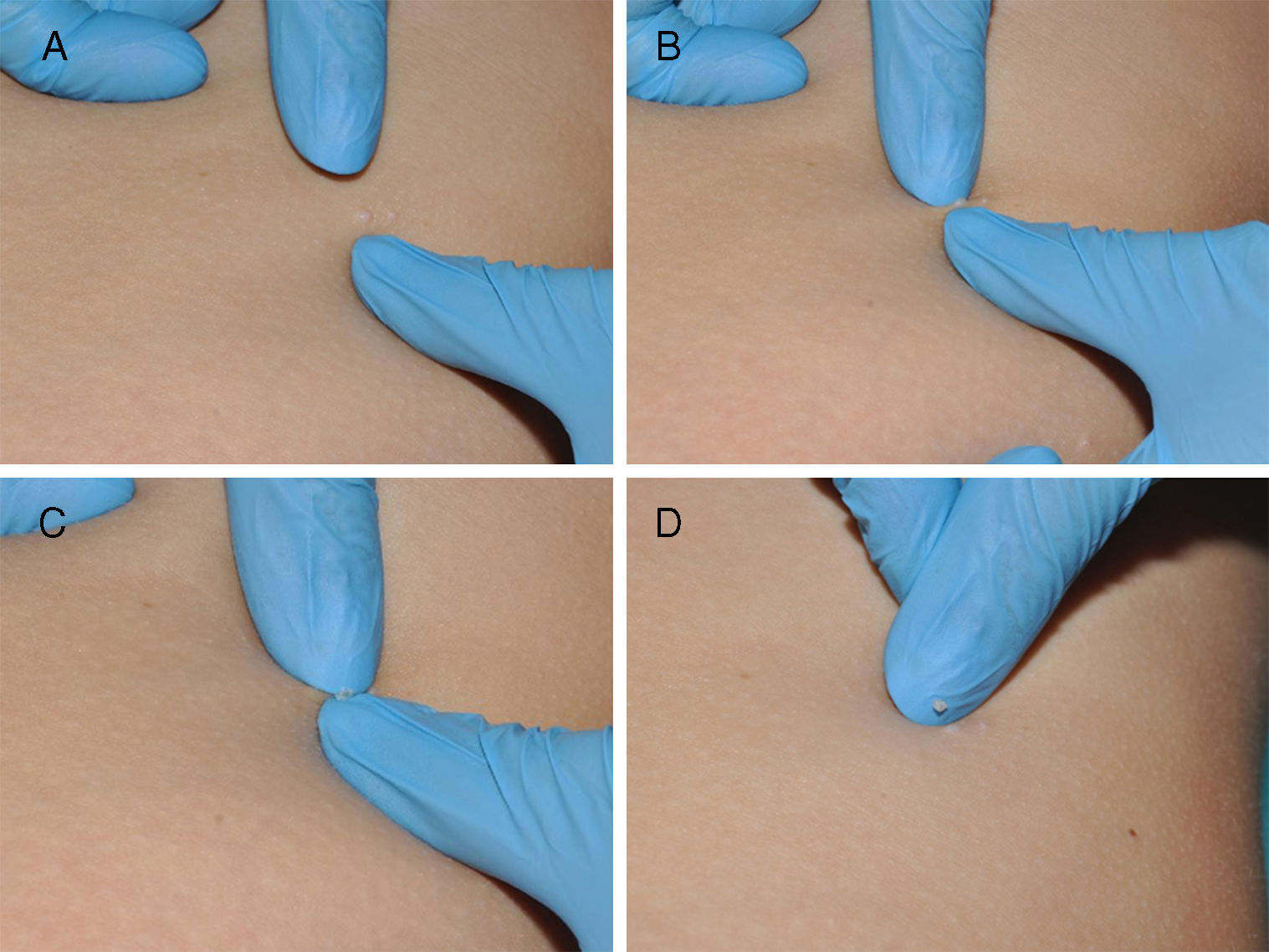
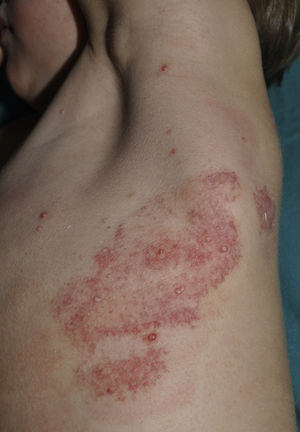
The umbilicated nucleus of the lesion can be manually removed using the hands or any one of a variety of instruments, including a scalpel, lancet, insulin needle, slide, or forceps (Fig. 3). The resulting scarring is similar to that caused by curettage. This technique is of particular interest as it is simple and fast and can be learned by patients, family members, and caregivers and therefore performed at home.11
Trichloroacetic AcidTrichloroacetic acid causes tissue destruction by immediate chemical coagulation and superficial necrosis.18 It is used at concentrations of 20% and 35% and applied repeatedly on the center of the lesion until a white, frost-like covering forms. In a review of pediatric cases of facial MC treated with topical trichloroacetic acid, no irritation or marked pigmentary alterations were described, and patients reported only mild stinging during applications, which produced good clinical results.19 Adverse effects include pruritus in the treated area, irritation of the surrounding skin, ulceration, and scarring.18
Salicylic AcidSalicylic acid is a keratolytic agent sold at concentrations of 10% to 30%. A randomized controlled trial of treatment with 10% potassium hydroxide (KOH) or the combination of salicylic acid and lactic acid at 16.7% in 26 MC patients aged 2 to 12 years found no significant differences between groups after 6 weeks.20 Side effects included irritation, pruritus, a burning sensation, and peeling of the skin.
Hydrogen PeroxideHydrogen peroxide (HP) is a powerful oxidizing agent and antiseptic that can inactivate poxvirus in vitro.21 Treatment with HP, which is sold outside of Spain in a 1% cream, resulted in complete resolution of lesions in an 8-month-old patient with genital MC when applied at every diaper change for 1 week.22 The authors attributed the rapid resolution to greater exposure of the virus to HP because the skin was occluded by the diaper. In another study of 12 MC patients treated with 1% HP cream applied twice per day for 21 consecutive days, 67% attained full resolution without recurrence after 6 months of follow-up. Appropriate clinical trials are required to confirm the efficacy and safety of HP for the treatment of MC in children.
CantharidinCantharidin is a vesicant agent produced by the beetle Lytta vesicatoria.23 When applied to the skin, this phosphodiesterase inhibitor produces an intraepidermal blister that rarely leaves a scar owing to its superficial location.17 It is used at concentrations of 0.7% to 0.9%, and after application should be left in place for 2 to 4hours without occlusion and subsequently removed with soap and water.17 Other authors have proposed that in cases of resistant lesions cantharidin should be allowed to dry for 5 to 10 minutes and then occluded with adhesive tape.24 The treatment can be repeated at intervals of 1 to 4 weeks. In a retrospective study of 300 children with MC who were treated with cantharidin, a cure rate of 90% was achieved with an average of 2.1 treatments.18 The treatment itself is painless, but within 24 to 48hours painful blisters form, bringing the added risk of secondary superinfection. Cases of lymphangitis with lymphedema following cantharidin treatment have also been reported.25 Given these risks, cantharidin is not recommended for MC of the face or anogenital region.16
Potassium HydroxidePotassium hydroxide (KOH) is an alkali that penetrates and destroys the skin by dissolving keratin. It is used in aqueous solution at concentrations of 5% to 20%, and applied to MC lesions once or twice per day.20,26 In a prospective trial in which 35 children with MC lesions received twice-daily treatments with 10% KOH aqueous solution, complete lesion resolution was observed in 32 of the patients.27 Applications were discontinued in 3 patients due to severe stinging and secondary infection. The efficacy of KOH has been compared with that of other MC treatments. No significant differences were reported in a trial comparing the efficacy of cryotherapy with that of 10% KOH in solution for the treatment of MC.26 However, the higher cost and secondary local effects of cryotherapy would tend to favor the use of KOH. Another study found that 10% KOH and 5% imiquimod cream were equally effective, but that KOH had a faster onset of action.28 Finally, a third study compared 10% KOH administered once per day with salicylic acid and lactic acid in combination, finding they were equally effective in the treatment of MC.20 Because 10% KOH treatment is noninvasive, efficacious, and can be applied at home, many authors consider it to be the first line of therapy.29
CryotherapyThe application of liquid nitrogen at –196°C induces the formation of intracellular and extracellular ice crystals, which cause tissue destruction and changes in the cell membrane and circulation in the skin.18 Liquid nitrogen is applied with a cotton swab or a portable sprayer for 10 to 20 seconds in 1 or 2 treatment cycles at intervals of 1 to 3 weeks. In a prospective study that recruited 74 children with MC the clinical efficacy of weekly cryotherapy was compared with that of 5% imiquimod administered 5 times per week.30 After 16 weeks of treatment, complete resolution was observed in 100% of patients treated with cryotherapy and 91.8% of those treated with imiquimod, but the difference was not statistically significant. While cryotherapy can be easily and rapidly administered, it is very poorly tolerated in young children. Other disadvantages include the formation of blisters, the possibility of scarring, and residual hyper- or hypopigmentation.
Laser TherapySome authors consider carbon-dioxide (CO2) laser therapy to be a faster and less traumatic approach than curettage. However, in a study of 6 patients treated with CO2 laser, hypertrophic scars and keloids were observed in 70% of treated patients, and therefore its use in children is not recommended.31 Some authors consider pulsed dye laser therapy to be particularly useful in children with resistant lesions. Because only a single treatment cycle is required in most cases, anxiety associated with repeated treatments is minimized.32 However, this treatment modality is expensive and sometimes requires local anesthesia. The adverse effects of this type of laser therapy include localized pain and discomfort, edema, and pigmentary changes.
ImmunotherapyImmunotherapeutic methods are based on the stimulation of a cellular and/or humoral immune response that can eliminate the viral infection.
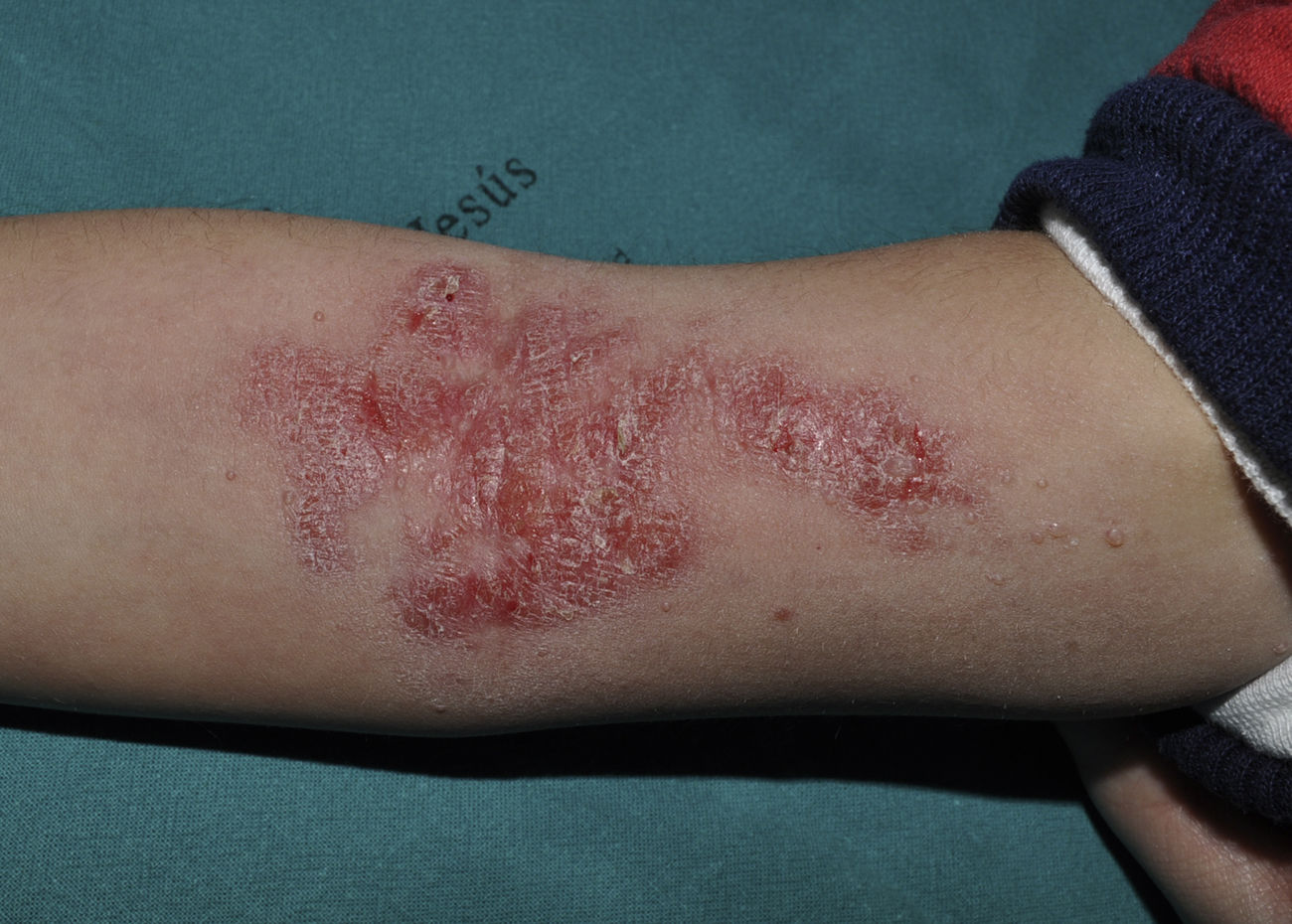
ImiquimodImiquimod, an agonist of toll-like receptor 7, binds to this receptor, activating the innate immune response and inducing the synthesis of interferon-α, interleukin (IL)-1, IL-5, IL-6, IL-8, IL-10, and IL-12, and IL-1 receptor antagonist, among other factors. Imiquimod's antiviral and antitumor effects are mediated by both the adaptive and innate immune systems.33 It is available in a 5% cream to be applied at night, left for 8hours, and rinsed off in the morning. Some authors recommend daily application while others suggest 3 treatments per week.34 In one study in which children with MC were treated 3 times per week for 16 weeks with 5% imiquimod cream, complete resolution of MC was observed in 69%.35 The most frequent local adverse effects were erythema, pruritus, stinging, and pain, which in some cases was intense (Fig. 4).
CimetidineOral cimetidine is an antagonist of H2 histamine receptors. It exerts immunomodulatory effects by stimulating delayed hypersensitivity. In a clinical study of 13 children of less than 10 years of age who were treated with 40mg/kg of oral cimetidine once per day for 2 months, complete lesion resolution was observed in 9 of 13 patients.36 The authors concluded that cimetidine was an easy to apply, effective, and painless alternative for treating facial, widespread, or recurrent MC in immunocompetent children. However, in a double-blind trial comparing placebo treatment with oral cimetidine (35mg/kg) administered once per day for 12 weeks in MC patients aged 1 to 16 years, no statistically significant differences were observed between the placebo and treatment groups.37 Based on this finding, the authors proposed that the efficacy observed in other studies may in fact be the result of spontaneous lesion resolution. Side effects of oral cimetidine are rare but include nausea, diarrhea, rash, and dizziness.36
CandidinCandidin, a substance derived from the purified extract of Candida albicans, is usually used to treat warts38 but has been proposed as a treatment option for MC.39 It is administered intralesionally either undiluted or at a concentration of 50% in lidocaine. The dose administered corresponds to 0.2 to 0.3mL of the antigen. In one retrospective study of 29 MC patients under the age of 17 who were treated with 0.3mL of intralesional candidin the global response rate was 93%, and complete and partial responses were observed in 55% and 37.9% of patients, respectively.40 Most side effects were minimal, but pain at the site of injection was experienced by 4 patients. In another retrospective review of 25 MC cases treated with intralesional candidin, complete resolution was observed in 14 (56%) cases, a partial response in 7 (28%), and no clinical improvement in 4 (16%).39 The advantages of immunotherapy in the treatment of MC include the induction of a memory immune response to MC, the potential to induce a generalized response that leads to resolution of untreated lesions in anatomically distant sites, and the lack of adverse effects.40 However, candidin, which is not commercially available in Spain, is scarcely used in clinical practice.
Silver NitrateSilver nitrate is prepared with 0.2mL of a 40% aqueous solution of silver nitrate and 0.05g of flour. This semitransparent mixture is placed in the center of the lesion. After 24hours a dark crust begins to appear, and after about 14 days the MC lesion falls off. Treatment of 389 consecutive MC patients with 40% silver nitrate resulted in a cure rate of 97.7% and caused no scarring.41 This simple, inexpensive procedure is painless and causes few adverse reactions such as pain, stinging, erythema, chemical burns, or residual hyperpigmentation.42
Antimitotic TherapiesCidofovirCidofovir is a nucleotide analogue of deoxycytidine monophosphate. Although its mechanism of action remains unclear, it is known to inhibit viral DNA polymerase, therefore blocking the synthesis of viral DNA. Cidofovir can be administered intravenously (5mg/kg/wk for 2 weeks followed by 5mg/kg once every 2 weeks) or topically (1%–3% cream or gel, applied daily).43 Several studies have described the successful use of intravenous or topical cidofovir for MC resistant to other treatments.44 However, this drug is expensive and further studies are required to determine its efficacy and safety in children.
Other TreatmentsThe evidence base supporting several treatments of scarce efficacy is weak, but they are harmless and generally well accepted by parents and caregivers. Such treatments may be useful in patients with multiple resistant lesions for whom active treatment is sought. These treatments include local hyperthermia,45 occlusion with adhesive tape,46 and the topical application of Polypodium leucotomos extract, immunoferon,47 zinc oxide,48 azelaic acid, and certain natural products such as essential oil of Australian lemon myrtle leaves.49
Wait and SeeBecause MC is benign and self-limiting, a wait-and-see approach is reasonable. The time to resolution of MC varies. In a prospective community cohort study the average time to resolution of MC lesions in 306 British MC patients aged 4 to 15 years was 13.3 months.50 Thirty percent had not resolved at 18 months, and 13% remained unresolved at 24 months. However, many parents will not accept an indeterminate estimate of time to resolution and fear the potential risk of spread or transmission to other children.23 Moreover, in some cases the disease can be uncomfortable or stigmatizing. One survey found that parents were twice as likely as their children with MC to express significant concern about the disease.6 Parents’ concerns related to the clinical manifestations of MC (scarring, spread, itching, and pain) and the discomfort caused by available treatment methods. However, the same study found that infection did not significantly affect daily activities, quality of life, or individual productivity in school.
Our Approach to the Treatment of MCBecause MC tends to resolve spontaneously, we often choose to wait and see, especially if the lesions are asymptomatic and the parents, for whatever reason, prefer to let the disease run its natural course. If the lesions cause discomfort, are located in very visible areas, or lead to the child's exclusion from school activities, we choose active treatment.
The choice of treatment depends on the number of lesions, their location, potential adverse effects, parental preferences, and the physician's experience. In general, we avoid any procedures that cause intense pain or are associated with a significant risk of scarring (eg, cryotherapy or laser therapy).
Manual extrusion of the molluscum body using the fingers is a simple and inexpensive technique, and is ideal when the affected child has few lesions and is afraid of surgical instruments like curettes, scalpels, or clamps. Curettage is probably the most effective technique, but requires skill and patient collaboration, which is often lacking (particularly in cases that require repeated treatments or involve facial lesions). Topical EMLA can minimize the pain, but does nothing to diminish fear in children. Moreover, topical anesthesia is difficult to apply in certain locations such as the eyelids. Although sedation of the patient is a possibility, this option is reserved for very specific circumstances.
In Spain, KOH is sold at concentrations of 5% and 10% and can be applied at home. Both formulations are suitable for the treatment of patients with a large number of lesions or lesions on the trunk and extremities. KOH is also useful when children do not collaborate by staying still for treatment or when parents are reluctant to allow curettage. We tend not to use any of the other topical products available in Spain because in our experience they cause considerable local irritation and show relatively poor efficacy. Furthermore, very few are formally indicated for treating MC in children.
ConclusionAlthough MC is one of the most common viral skin diseases in children, there is no consensus as to the treatment of choice or whether patients should even be treated. No scientific evidence clearly favors a specific treatment for MC. According to the newly developed Strength of Recommendation Taxonomy for rating the quality, quantity, and consistency of evidence for therapies, support for MC management options would fall at level B at best, indicating a lack of consistent, high quality evidence available.42,51
In principle, MC should be treated using modalities that cause minimal pain and scarring. It is also important to determine the most appropriate treatment for each particular case.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Gerlero P, Hernández-Martín Á. Actualización sobre el tratamiento de moluscos contagiosos en los niños. Actas Dermosifiliogr. 2018;109:408–415.