Lentigo maligna is an in situ cutaneous melanoma that arises in sun-damaged skin. Its most common presentation is a progressive, slow-growing, irregularly pigmented spot on the face of older patients. Although the exact percentage of lentigo maligna that progresses to invasive tumors is unknown, it is thought to lie between 2% and 5%. Both the clinical and histologic diagnosis of lentigo maligna can be challenging, especially in patients with early-stage or atypical disease. Treatment also holds challenges, because lesions are located in highly visible areas and are often large. Surgery can thus compromise cosmetic and sometimes functional outcomes. We review clinical and histopathological findings that can facilitate the diagnosis of lentigo maligna. We also examine treatment options, with a focus on surgery.
El lentigo maligno es un melanoma cutáneo in situ que asienta en zonas con daño solar acumulado. Su presentación más habitual es como una mancha irregularmente pigmentada de crecimiento lento y progresivo localizada en la cara de un paciente añoso. Aunque el porcentaje real de casos de lentigo maligno que evoluciona a formas invasoras es desconocido, se calcula que supone entre un 2 y un 5% de los casos. Tanto el diagnóstico clínico como histopatológico del lentigo maligno puede suponer un reto, especialmente en casos precoces o atípicos. Su tratamiento también puede suponer un desafío por su localización en áreas muy visibles y por su tamaño frecuentemente considerable, lo que tiene implicaciones estéticas y ocasionalmente también funcionales derivadas de la cirugía. En este trabajo revisamos las claves clínicas e histopatológicas para facilitar el diagnóstico del lentigo maligno. También revisamos las opciones de tratamiento con especial atención a la cirugía.
Lentigo maligna (LM) is a form of in situ cutaneous melanoma that typically arises in areas of significant sun-damaged skin; it is particularly common on the nose and cheeks of older people. LM that has invaded the dermis or beyond (i.e., that is no longer in situ), is known as lentigo maligna melanoma (LMM). Similarly to in other forms of melanoma, prognosis is influenced by Breslow thickness, ulceration, and other histologic and clinical characteristics.1 LM accounts for approximately 10% of all melanomas. It has a longer in situ growth phase and may not become invasive for decades. According to a 1987 study, just 5% of LMs become invasive, and this percentage drops to 2.2% for tumors diagnosed after the age of 65 years.2 These figures, however, may not be reliable, as they are based on LM and LMM prevalence studies. A similar, more recent study, reported that 2% to 2.6% of patients diagnosed with LM developed LMM over a period of 25 years, and that 90% of these tumors were in the same area as the original tumor.3 Other authors have estimated a 3.5% annual risk and a mean time to progression of 28.3 years.4 The real risk of progression, however, is difficult to ascertain, but it exists and should not be underestimated.
Diagnosis of LM is often delayed for a number of reasons. First, many patients may postpone visiting a doctor until the lesion changes color, grows, or starts to ulcerate or bleed, as they are typically older patients with evident signs of sun damage who see the LM as “just another spot”. In other cases, it may be the general practitioner or even the dermatologist who misses the diagnosis, as early clinical phases of LM can easily be confused with other lesions, such as sun lentigines. Finally, the diagnosis may be missed by the pathologist because very early histologic changes are subtle and can be difficult to differentiate from actinic melanocytic hyperplasia, a characteristic finding in sun-damaged areas. In more advanced phases and in the absence of clinical-pathologic correlation, the lesion can be confused with junctional dysplastic nevus.5
The aim of this study was to review and update the most important aspects of LM and highlight the clinical, and in particular, the histopathologic findings that can help minimize diagnostic delays.
Epidemiology, Etiology, and Pathogenesis of LMAccording to statistics from the Surveillance, Epidemiology, and End Results (SEER) program, LM/LMM, with an annual incidence of 1.37 cases per 100000 population, is the second most common clinicopathologic subtype of melanoma in the United States, preceded only by superficial spreading melanoma (SSM).6 The respective incidence in Whites (not counting Hispanics) is 1.87 cases per 100000 population a year. Rates are much lower in Hispanics, Asians, and Blacks, with a respective incidence of 0.23, 0.06, and 0.02 cases per 100000 population a year.6 Because of the association with cumulative sun damage, LM/LMM is much more common in patients older than 65 years. According to the same SEER source, the annual incidence of LM/LMM in adults over 65 years was 10.11 cases per 100000 population in non-Hispanic whites, 1.38 cases per 100000 population in Hispanic whites, 0.16 cases per 100000 population in Asians, 0.33 cases per 100000 population in Blacks, and 7.69 cases per 100000 population in the overall population.6 In Spain, the respective incidence among men and women has risen from 0.25 and 0.18 cases per 100000 population in 2000 to 0.68 and 0.63 cases per 100000 population.7 According to data on 10581 patients with invasive melanoma in the National Melanoma Registry of the Spanish Academy of Dermatology and Venereology (AEDV), LMM is the third most common subtype of melanoma (8.9%) in Spain, behind nodular melanoma (19.8%) and SSM (65.1%).8
Whiteman et al.9 postulated the presence of at least 2 etiopathogenic pathways for the development of cutaneous melanoma. The first would involve individual susceptibility to melanocyte proliferation, expressed phenotypically as a high number of melanocytic nevi linked to skin damage caused by intense intermittent UV radiation exposure. The second pathway would be cumulative skin damage induced by chronic UV radiation exposure. This would involve a significant degree of solar elastosis in the skin in which the melanoma develops and a phenotype marked by an increased prevalence of actinic keratoses and nonmelanoma skin cancer. According to the latest edition of the World Health Organization (WHO) Classification of Skin Tumors, degree of solar elastosis is a defining feature of melanoma subtypes.10 LM is the predominant subtype in this pathway, and accordingly, it is preferentially found on the skin of the head or neck, or, to a lesser extent, the arms. Risk factors that have been linked more closely to LM than SSM are solar lentigines and a personal history of skin cancer. Melanocytic nevi and a history of burns have a significantly smaller influence in LM than in SSM, and in both subtypes, risk is increased by actinic keratoses and fair, UV-sensitive skin.11–13 Risk of melanoma is also higher in geographic areas with high levels of solar radiation14 and in individuals with cumulative sun exposure (mostly men).15
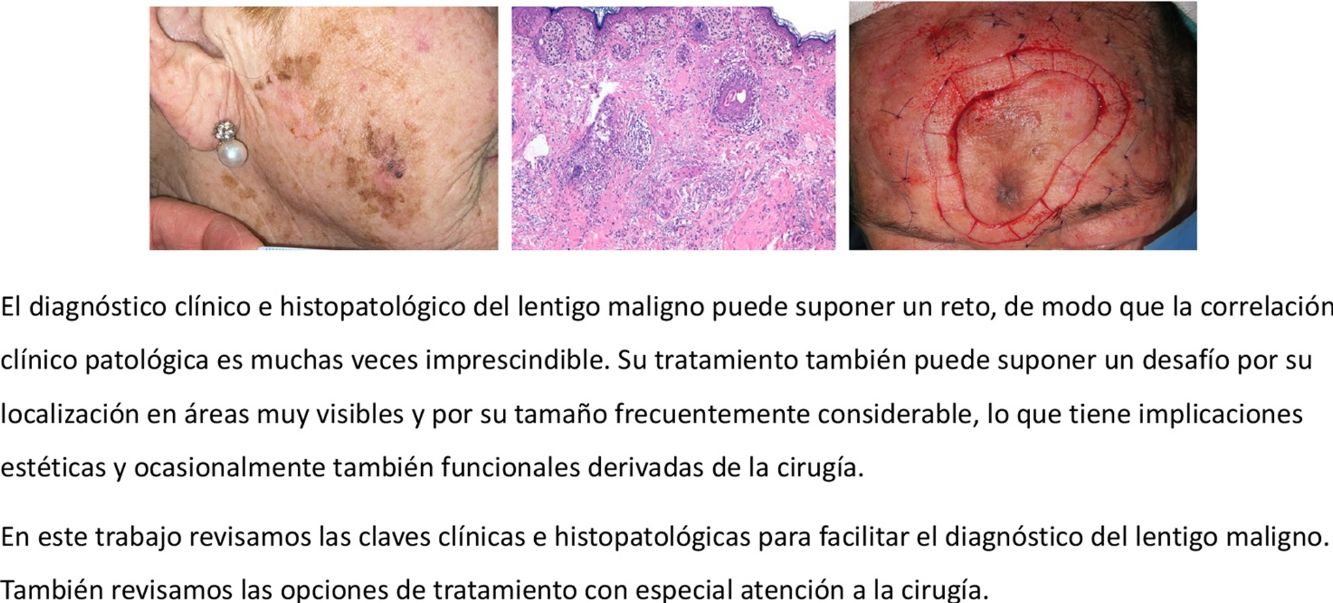
Clinical CharacteristicsLM typically manifests as an irregular pigmented patch on the head and neck of older patients. The most common location is the cheek. The lesions are normally brown, with a variety of tones ranging from light brown to black. They have a heterogeneous pigmentation and edges that are often (Fig. 1A) but not always (Fig. 1B) ill defined. Like other forms of melanoma, LM may also contain pink, reddish, or whitish areas, generally in the context of regression.16 In the absence of evident signs of histologic regression, LM with predominant white or reddish areas (Fig. 1C, D) can be difficult to diagnose clinically17–19 and may be confused with vitiligo, Bowen disease, or eczema.5 Observation of characteristic areas of LM close by can be key to considering LM in the clinical differential diagnosis. It is therefore strongly recommendable to biopsy any dyschromic area adjacent to an LM, even if it is white or reddish in color and exhibits no signs of melanoma, as it could be an extension of the lesion.20 In the absence of characteristic findings, LMs with exclusive white or reddish pigmentation are very difficult to diagnose. They should, however, be contemplated whenever a localized hypopigmented or erythematous area is observed in a patient with extensive sun-damaged skin. LM can grow to a considerable size without becoming invasive. Lesions affecting periorificial areas, such as the mouth and eyes, can spread to nearby mucosal surfaces.5
Clinical features of lentigo maligna. A, Large patch with ill-defined edges showing different shades of brown with reddish and black areas on the right cheek. B, Brownish patch with black areas and ill-defined edges on the left ear. C, Red patch with indistinct borders on the right cheek. Note the scar in the right nasolabial fold from a previous lentigo maligna treated with conventional excision. D, White patch in the lower half of a lentigo maligna on the left cheek. The lesions in C and C showed no histologic evidence of regression.
The main entity to consider in the clinical differential diagnosis of LM is solar lentigo. This normally has a more uniform color and grows more slowly. Dermoscopy can be helpful, as LM has a number of characteristic features, such as asymmetric pigmentation, obliterated follicular openings, rhomboidal structures, an annular-granular pattern, and a gray pseudonetwork.21 These last 2 findings are less specific. Other dermoscopic features of LM are reddish rhomboidal structures, a vascular network, and an image known as a circle in a circle.22 Histology is essential for establishing a definitive diagnosis in equivocal cases. Pigmented actinic keratosis is less common, but should be considered in the clinical differential diagnosis. This lesion characteristically has a rough texture, and dermoscopy is helpful.23,24 Again, histology is required in cases of uncertainty. Some authors have proposed using an inverse dermoscopic approach to identify a potential LM, which involves ruling out the characteristic features of the benign lesions described above.25
Although LM characteristically affects older patients, midfacial lesions are not uncommon in patients aged around 50 years, especially fair-skinned patients with extensive cumulative sun damage. Less frequently, LMs can be observed on the dorsum of the forearms or on the upper back.26
An invasive tumor (LMM) should be suspected when an LM acquires a rough texture or becomes palpable. Progression is unpredictable, although it is more likely to occur on the scalp (3.4 times more likely than on the face or forehead) and when clinical changes are noticed by the patient (1.8 times more likely than when changes are not noticed).4
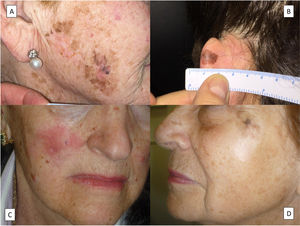
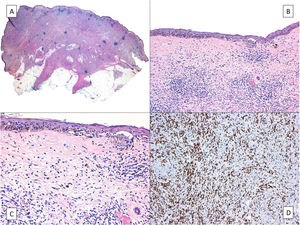
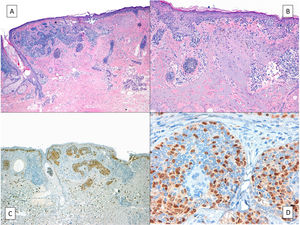
HistologyThe histologic diagnosis of LM is normally straightforward, except in very early stages, when findings are less evident. In such cases, a diagnosis is reliant on a high index of suspicion and careful histologic examination of biopsy specimens. In early lesions, when the proliferation of tumor melanocytes is not yet widespread, practically the only helpful finding is a slightly higher-than-normal number of irregularly distributed melanocytes (not equidistant to each other) in the basal layer, above a dermis with evident solar elastosis5 (Fig. 2A). The observation of occasional rows of melanocytes in the basal layer of the epidermis with involvement of the follicular epithelium can also be helpful (Fig. 2B).27 Marked atypia is not usually observed in these phases and there may be mild nuclear pleomorphism. This discreet, initial phase, is followed by a much more evident proliferation of melanocytes, with frequent rows replacing the basal keratinocytes. Junctional retraction clefts may sometimes be observed. Tumor melanocytes also invade the basal layer of the follicular epithelium, and it is not uncommon for these cells to extend beyond the follicular infundibulum to deeper portions of the follicle, such as the sebaceous glands and the inferior segment.28 At this stage, the dermis will exhibit solar elastosis (at times very marked) and some lymphocytic infiltration. Atypical melanocytes, if present, are another helpful finding. They can sometimes take the form of atypical epithelioid melanocytes, but at other times the atypia is not marked, with lymphocytoid, angulated, or spindle-shaped melanocytes. The vast majority of these morphologic forms will normally exhibit some nuclear pleomorphism, with different sizes and shapes, a variable degree of nuclear pyknosis, and prominent eosinophilic nucleoli.
A, A higher-than-normal number of irregularly distributed (not equidistant to each other) melanocytes in the basal layer over a dermis with marked solar elastosis. B, Moth-eaten appearance of a hair follicle due to neoplastic melanocytes in a lentigo maligna. C, Predominance of irregular, confluent junctional nests in a lentigo maligna with the appearance of a junctional dysplastic nevus. D, Melanocytic atypia, irregular nests, and above all solar elastosis, point to a diagnosis of lentigo maligna.
In our experience, junctional nests of atypical melanocytes are quite common in early phases of LM (Fig. 2C, D). Unfortunately, in the absence of integration with clinical findings, the lesions may often be histologically diagnosed as a “junctional nevus with atypia”, “dysplastic nevus”, or similar.5 This leads to unnecessary diagnostic delays, and in some cases, the tumor will have invaded the deeper layers by the time it is diagnosed, significantly worsening the patient's survival prospects. It is relatively straightforward to establish a correct diagnosis in such cases by integrating clinical and histologic findings. When a dermatologist biopsies an LM on the face, the main entity entertained in the differential diagnosis is a solar lentigo, or less often, pigmented actinic keratosis or lichenoid keratosis. Melanocytic nevus is almost never considered. A histologic diagnosis of melanocytic nevus when LM is clinically suspected is usually due to a lack of clinicopathologic correlation, underlining the importance of informing the pathologist of the location of the lesion and providing a clear clinical description to enable correct interpretation of the histologic findings. Dysplastic melanocytic nevi almost never occur on the face, and this diagnosis should be questioned even more in patients with significant sun-damaged skin, who are generally most prone to LM.5,29 Although nests of melanocytes may be histologically present at the dermoepidermal junction in both LM and dysplastic melanocytic nevus, in LM, they are usually irregular in shape and size, while in dysplastic melanocytic nevus, they tend to be more uniform. In addition, LM nearly always contains atypical lentiginous melanocytic proliferations in the basal layer, with frequent extension to the hair follicle and even the inferior segment. This does not occur in melanocytic nevus. Prominent underlying solar elastosis, a characteristic feature of LM, should also raise doubts about a diagnosis of junctional nevus. In true melanocytic nevi, melanocytes continue to fulfill their function of protecting against sun damage, forming what is known as the umbrella sign. Solar elastosis is therefore almost never seen in such cases.30 In brief, if the pathology report indicates a diagnosis of melanocytic nevus when the clinical impression is a solar lentigo or LM, there is a high likelihood that the diagnosis will be melanoma.
Actinic melanocytic hyperplasia, a characteristic feature of intensely sun-damaged skin, is probably the most difficult entity to distinguish from LM in the histologic examination. In actinic melanocytic hyperplasia, however, melanocytes tend to be equidistant from each other, and even though they may invade the hair follicle, they will never extend beyond the infundibulum (i.e., there will be no involvement of the sebaceous glands or the inferior segment). In addition, nests of melanocytes or melanophages in the superficial dermis are not uncommon in LM, but will never be seen in actinic melanocytic hyperplasia.5
The presence of solar elastosis in LM is somewhat of a controversial subject. Although prominent solar elastosis is a classic defining feature of LM, the literature does not specify the degree of elastosis that is necessary for a diagnosis to be made. The latest WHO Classification of Skin Tumors includes LM in the category of melanomas on chronically sun-damaged skin, which are defined as tumors with a high degree of elastosis, characterized by homogeneous clusters or masses of elastosis. Lentiginous forms of SSM, by contrast, are defined as tumors that may exhibit elastosis, but to a lesser degree.10 No studies, however, have provided biologic or clinical evidence of this difference, and no relevant implications for treatment have been observed.
Although the histologic diagnosis of melanoma should customarily be based on examination of a completely excised lesion, in LM, it is usually based on a partial biopsy specimen, since suspected LMs are usually already quite large. When performing a partial biopsy, it is important to choose a technique that will provide adequate material to allow for comprehensive assessment of the architecture and previously described alterations. Accordingly, a broad superficial shave biopsy or an elliptical excision biopsy, ideally with inclusion of clinically normal skin, is preferable to a punch biopsy.31 When LM is diagnosed in a partial biopsy, it should be recalled that there may be an invasive component in the rest of the lesion. According to one meta-analysis, 22% of all biopsied lesions initially diagnosed as melanoma in situ had an invasive component.32 In a more recent study of 255 cases, 9% of lesions initially diagnosed as LM had such a component.33 Presence of an invasive component is significantly associated with rows of melanocytes or artifactual clefts, particularly when more than 25% of melanocytes form nests.34 Other factors associated with invasion are pagetoid spread and a prominent inflammatory infiltrate.35 Invasion should also be suspected when dermoscopy shows irregular blotches, obliterated follicular openings, or a black color.36
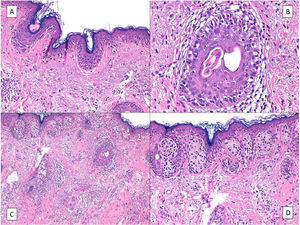
On rare occasions, LM can conceal an underlying desmoplastic melanoma (Fig. 3), with obvious prognostic and treatment implications.37 This possibility highlights the importance of palpating the lesion before biopsy, especially if a shave biopsy is to be performed.
Desmoplastic melanoma. A, Characteristic panoramic view of a desmoplastic melanoma invading the entire thickness of the dermis with extension into the hypodermis dotted with lymphoid nodules. B, The in situ component of the tumor is identical to that of lentigo maligna. C, Detailed view of the junctional component with moth-eaten epidermal basal layers due to melanocytes and a junctional nest of atypical melanocytes to the right of the image. D, Positive nuclear immunohistochemical staining with SOX10 in the dermal component.
Because LM can progress to an invasive, potentially fatal, melanoma, it must be treated. Treatment, however, is often challenging. On the one hand, margins are frequently ill defined due to the presence of subclinical extension, which is difficult to assess using conventional methods. This in turn makes it difficult to define safe surgical margins and assess response to nonsurgical treatments. On the other hand, surgery carries significant cosmetic risks, as LMs tend to occur on the face and are generally large by the time they are diagnosed.
Although a systematic review of surgical and nonsurgical treatments for melanoma in situ, including LM, did not find any high-quality evidence supporting the effectiveness of any of the treatments,38 guidelines recommend surgery as the treatment of choice.39,40 This is essentially because surgery is associated with lower recurrence rates and is the only treatment that allows full histologic examination of the surgical specimen. The main advantage of full histologic examination is the detection of potential invasive components in an LM diagnosed by partial biopsy.32,41 It also enables the detection of associated, clinically occult, tumors. Occult basal cell carcinomas (BCCs) have been described in this setting, although infrequently (Fig. 4).42,43 It is generally assumed that an LM that has colonized a BCC will not have extended beyond the epithelial component. In other words, it will still be an in situ lesion. The same applies to a melanoma in situ with adnexal extension. The metastatic potential of these tumors, however, cannot be completely ruled out, as very few cases of melanoma colonizing BCC have been described, and they are not without controversy.
A, Lentigo maligna (right half of the image) invading a basal cell carcinoma (left half of the image). B, Detailed view of the lentigo maligna with irregular nests of atypical melanocytes at the dermoepidermal junction and marked solar elastosis. C and D, Positive nuclear immunohistochemical staining with MITF highlighting the melanocytes in a lentigo maligna in situ; these cells are also dotted throughout the epithelial islands of the basal cell carcinoma.
The superiority of surgery as the treatment of choice for LM is supported by several meta-analyses32,40 that have compared different types of surgical and nonsurgical treatments, and shown much higher rates of local recurrence for the latter.40 Reported rates are 11.5% for radiotherapy, 24.5% for topical imiquimod 5%, and 34.4% for laser therapy, contrasting with just 2.8% for surgical treatments with exhaustive margin control (Mohs micrographic surgery [MMS] or staged surgery).44 Surgery with exhaustive margin control is the first-line approach for the treatment of LM for several reasons, including the difficulty of clinically delineating margins and the need to spare healthy tissue to minimize cosmetic defects.32 When this option is not available, margins of least 1cm should be obtained.20,45 Although the latest version of the National Comprehensive Cancer Network guidelines consider a margin size of between 0.5 and 1cm to be adequate, they recommend surgery with exhaustive margin control for LM. Several large studies have shown the inadequacy of 0.5-cm margins in this setting, as at least 14% of tumors had affected margins, and complete excision rates of over 95% were only obtained with a margin of at least 0.9cm.20,45
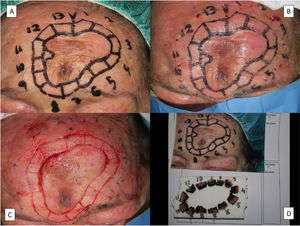
A range of surgical techniques exist to evaluate margins and ensure, using a variable number of steps, complete excision of LM. Numerous studies have shown good results for MMS using frozen sections. Slow MMS, or slow Mohs with permanent formalin-fixed sections, however, should be considered the technique of choice for evaluating margins in any melanocytic lesion.46 This technique is generally performed using a 90° rather than a 45° incision to facilitate assessment of the lateral margins, which is the primary reason for using MMS in LM. With slow Mohs, the surgical bed is normally marked with orienting sutures that match those on the surgical specimen sent for analysis. The inflammation caused by these sutures, however, can make it difficult to interpret subsequent Mohs steps, if required.47 To overcome this problem, we use a modified version of the technique that involves placing the orienting sutures at least 1cm from the surgical margin (Fig. 5).
Removal of lentigo maligna by slow Mohs micrographic surgery. A, Presurgical delineation of tumor, the 5-mm margin, and design of the margin segments to be analyzed. B, Nylon sutures used to mark the sections at a distance of 1cm from the surgical margin. C, Prepared sections and orienting suture in section 1 for subsequent processing. D, Sections duly arranged, numbered, and pinned to a cork. All the margins except the surgical margin, which is what is analyzed in slow Mohs micrographic surgery, are stained with India ink.
Immunohistochemistry is an important tool for differentiating LM from surrounding skin, which often contains atypical melanocytic hyperplasia. The most widely used stains for this purpose are MART-1/Melan-A, MiTF, and SOX10.48,49 Melanocytes can be both identified and quantified using MiTF and SOX10 due to their selective nuclear staining of melanocytes. MART-1/Melan-A is a less specific stain that can also stain melanin-laden keratinocytes in areas with significant actinic damage. There are several key features that help distinguish actinic melanocytic hyperplasia from LM. Essentially, in the former, 1) melanocytes in the basal layer are distributed regularly, at similar distances to each other, 2) melanocytes may invade the follicular infundibulum but not the sebaceous glands or the lower segment, 3) nests are never present, and 4) melanophages are never present. The main features for identifying a positive margin are nests containing at least 3 melanocytes, confluence of at least 10 melanocytes in the basal layer of the epidermis, pagetoid spread to the upper half of the epidermis, significant melanocytic atypia (with large atypical nuclei or pleomorphism), and follicular involvement beyond the infundibulum. In their absence, a high density of melanocytes can also indicate margin involvement. This last finding should be interpreted with the context of the location of the tumor, as different anatomic sites have different densities. Some authors have proposed using contralateral skin as a negative control.50 Although rapid immunohistochemistry assays have been described for the staining of frozen tissue sections using MART-1/Melan-A and MiTF, surgical techniques evaluating conventionally processed tissue are more common. Two techniques have been proposed to ensure complete excision based on full assessment of the lateral margins and examination of the center of the specimen to rule out an invasive component. Both techniques are associated with recurrence rates of less than 5% at 5 years. The first involves complete excision of the lesion with mapping and marking of the lateral margins to check for malignancy in each segment and simultaneous, independent inspection of the center of the specimen.51,52 The second involves excising the margins for mapping and analysis and leaving complete excision to the last surgical step, when the defect is also repaired. This step is performed on confirmation of clear margins. The technique is known by different names, as there are slight modifications to the procedure used. The most widely accepted term is the spaghetti technique,53 but other terms are the Johnson square technique, the Tübingen technique, and the contoured technique.54–56 Pain control and the prevention of infections are important with the first technique, as the open surgical wound must be kept immobilized in order to accurately locate any positive margins detected. One option is to use biosynthetic dressings that reduce both pain and infection risk.
Either of the above techniques can be aided by Wood's light,57 dermoscopy,58 hyperspectral imaging,59 or in vivo confocal microscopy,60–64 or a combination of 2 of these. These tools help delineate margins and decrease the number of surgical stages and total operating time required.
Once negative margins have been obtained, the surgical defect should be closed by direct suturing or, if not possible, grafting or healing by secondary intention. Flaps and plasty repair should be avoided wherever possible to facilitate the detection and treatment of potential local recurrences. It is important to note that late recurrence is common in LM, with tumors relapsing on average 57 to 71 months after surgery depending on the series.65,66 Long-term follow-up, extending beyond 5 years, is therefore necessary.
A number of nonsurgical treatments exist for LM. Choice of treatment should be guided by effectiveness, risk of progression to LMM, lesion size and location, and the patient's health. There are no established criteria to guide treatment decisions, but several factors can help choose the best option for each case. Therapeutic abstention is a valid option for patients with poor general health and life expectancy, especially if the LM is large. When life expectancy is sufficiently long, but the patient is not a candidate for the previously described surgical techniques because of their general health, it would seem reasonable to treat the lesion nonsurgically.
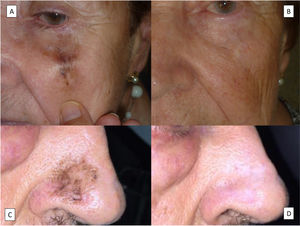
Radiotherapy is the nonsurgical modality with the highest response and lowest recurrence rates (Fig. 6A, B). It is, therefore, the treatment of choice when surgery is not feasible.67–69 One of the main advantages of radiotherapy over other nonsurgical treatments is that it will treat invasive components if present. Type of radiotherapy, total dose, and fractionation schedules vary according to the series. Doses of 42 to 160Gy administered in 3 to 13 fractions every 1 to 4 days have been used.70 For superficial radiotherapy, patients have been administered doses of 35 to 57Gy in 5 to 23 fractions every 1 to 4 days.70 If detected, invasive components should be excised together with 0.5 to 2cm of perilesional skin.71 The above-mentioned imaging techniques can help delineate the treatment field. Another option is to perform histologic mapping using partial biopsies around the clinical lesion. The most comprehensive study on the use of radiotherapy in LM to date showed a complete response rate of 97% over a 10-year follow-up period. Just 2 of the 70 lesions recurred, and no differences were observed between radiotherapy and surgery for patient-rated cosmetic outcomes.69
The second nonsurgical option is imiquimod (Fig. 6C, D). Imiquimod is very popular among dermatologists due to its excellent cosmetic results and the extensive experience with its use in the treatment of actinic keratosis and BCC. It is most effective when applied for 6 to 7 days a week up to at least 60 applications, although, as with actinic keratosis and BCC, the regimen can be modified depending on inflammatory response.72,73 Imiquimod, however, should be used with caution in this setting, as this is an off-label application and there have been several reports of progression to LMM during its use. Progression has been reported in at least 2% of cases, but the mean follow-up was just 22 months, and while most progressions occurred in the first year, 1 LMM was detected at 25 months.72 Recurrence during treatment with imiquimod should be suspected if a brownish pigmentation, in particular with a perifollicular distribution, is observed during the first 2 years of follow-up.74 Imiquimod can also be used an adjuvant treatment when clear margins cannot be obtained surgically. Similar recurrence rates, ranging from 6% to 9% after a mean follow-up of 21 to 35 months, have been observed in both scenarios.75
Finally, it important to recall that long follow-up periods (probably spanning at least 10 years) are required for LM, because many recurrences occur late (>5 years). A recent follow-up study of 100 LMs and LMMs with a Breslow thickness of less than 1mm treated by staged excision reported 4 local recurrences after a mean of 71 months, leading the authors to conclude that their study, and most others, had probably underestimated the risk of LM/LMM recurrence.
Conflicts of InterestThe authors declare that they have no conflicts of interest.