Apremilast is a therapeutic option in patients with psoriasis and/or psoriatic arthritis who do not respond to conventional systemic therapy.1 Based on the results of clinical trials, the clinical profile thought to benefit the most from apremilast is that of patients with moderate plaque psoriasis, and it is an alternative for involvement of special sites (scalp, nails, or palms and soles).2 Because studies in clinical practice are rare, we performed a retrospective, descriptive, observational study to assess the effectiveness and drug survival of patients with psoriasis and/or psoriatic arthritis treated with apremilast at our hospital.
A total of 65 patients were enrolled in the study between January 2015 and February 2020. Most received the treatment in a single-drug therapy regimen (53/65; 81.5%). Of the 65 patients, 55 had plaques (84.6%), 4 had palmoplantar nonpustularl psoriasis (6.2%), 1 had palmoplantar pustular psoriasis (1.5%), 8 had involvement of the scalp (10.8%), and 9 had nail involvement (13.8%). In terms of baseline severity, 17 patients (26%) had mild psoriasis (Psoriasis Area and Severity Index [PASI]≤3), 26 (40%) had moderate psoriais (PASI of 4–12), and 22 (34%) had severe psoriasis (PASI≥13). Apremilast was prescribed owing to associated comorbidities in 31 patients (47.7%), lack of response to systemic treatments in 22 (33.8%), abnormal liver biology in 5 (7.7%), intolerance or contraindication of systemic treatments in 4 (6.2%), active or latent infection in 2 (3.1%), and a history of cancer in 1 (1.5%). The patients’ other demographic and clinical variables are summarized in Table 1.
Results of the Variables Analyzed in Our Series.
| Patient clinical characteristics (n=65) | |
|---|---|
| Mean age, y±SD | 56.7±1.8 |
| Sex, male/female, % | 41.5/58.8 |
| Comorbidities, n (%) | |
| • Psoriatic arthritis | 20 (30.8) |
| • Hypertension | 13 (20) |
| • Type 2 diabetes mellitus | 7 (10.8) |
| • Dyslipidemia | 14 (21.5) |
| • Ischemic heart disease | 3 (4.6) |
| • Neoplasms | 7 (10.8) |
| • Renal failure | 2 (3.1) |
| • Fatty liver disease | 6 (9.2) |
| Previous treatments, n (%) | 55 (90.2) |
| • Phototherapy | 12 (18.5) |
| • Methotrexate | 42 (70) |
| • Acitretin | 37 (57.8) |
| • Ciclosporin | 8 (12.3) |
| • Leflunomide | 1 (1.5) |
| • Adalimumab | 2 (7.7) |
| Baseline BMI, kg/m2mean±SD | 27.79±5.62 |
| Baseline PASI, mean±SD | 10.06±6.98 |
| Baseline DLQI, mean±SD | 13.89±5.97 |
| Effectiveness by groups Week 24 Week 52 | |
| PASI50, n (%) | 21/32 (65.63) 14/16 (87.5) |
| Reduction in palmoplantar PGA≥2 points | 2/3 (66.7) - |
| Psoriatic arthritis | |
| • Partial response | - 10/20 (50) |
| • Complete response | - 5/20 (25) |
| • No response | - 5/20 (25) |
| NAPSI50, n (%) | 5/9 (55.6) 7/9 (77.8) |
| Safecty (n=65) | |
| 1 or more adverse effects, n (%) | 14 (21.5) |
| • Gastrointestinal (nausea, diarrhea) | 10 (15.4) |
| • Headache | 3 (4.6) |
| • Upper respiratory tract infections | 1 (1.5) |
| Treatment interruption (n=65), n (%) | |
| • Lack of response | 23 (35.4) |
| • Adverse effects | 3 (4.6) |
| • Loss to follow-up, poor adherence, or others | 2 (3.1) |
Abbreviations: PASI indicates Psoriasis Area and Severity Index; NAPSI, Nail Psoriasis Severity Index; PGA, Physician Global Assessment; DLQI, Dermatology Life Quality Index; BSA, Body Surface Area.
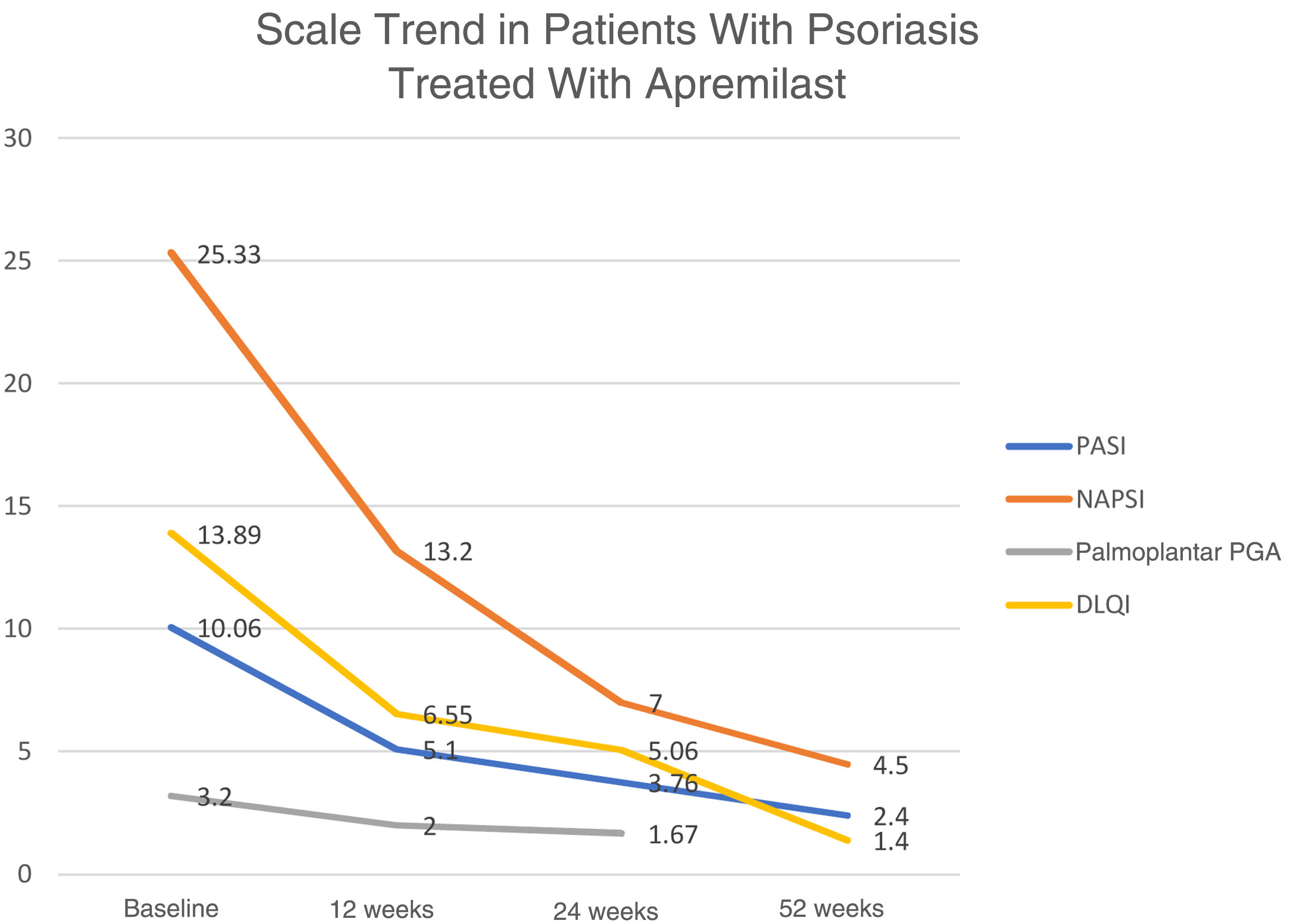
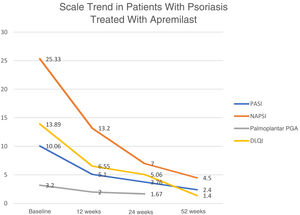
With regard to effectiveness in patients, data available at week 24 and week 52 show that 21/32 (65.6%) and 14/16 (87.5%), respectively, achieved a PASI score of 50. In the group of patients with psoriasis of the scalp, it was only possible to collect data on a single case that achieved a reduction of 3 points on the Physician Gloal Assessment (PGA) baseline score at week 24. The trend in the mean of the different scores used to measure the severity and repercussion of psoriasis (PASI, Nail Psoriasis Severity Index [NAPSI], palmoplantar PGA, and Dermatology Life Quality Index [DLQI]) shows a gradual reduction in weeks 12, 24, and 52 (Fig. 1).
In terms of safety, the most frequent adverse effects were gastrointestinal (10/65; 15.4%). Treatment was suspended in 28 (43.1%) of the patients for different reasons (Table 1). Drug survival after 1 year was 63.7%, with a mean (SD) continuation time with the drug of 152 (51) weeks. The Cox regression analysis model showed no statistically significant increase in the risk of treatment interruption for any of the following variables: baseline psoriasis severity, body mass index (BMI), sex, or prior systemic treatment.
The profile of the patients in our study differs from that of pivotal clinical trials3,4 and is similar to that of other clinical-practice studies. Del Alcázar et al.5 recently published the largest study in real-world clinical practice to date (n=377). If we compare the baseline characteristics of that study with ours, we see a population with similar age, baseline PASI, BMI, and mean DLQI. Our study has a slightly smaller proportion of men (41.7% vs. 47%) and a smaller percentage of patients who received biological therapy before the apremilast (7.7% vs. 21.7%). We also find that the proportion of patients who achieved PASI50 after 52 weeks was greater (87.5% vs. 77.4%), with a lower rate of adverse effects (21.5% vs. 47%). The percentage of patients who interrupted treatment was higher in our study (43.4% vs. 23.9%). Lack of effectiveness was the main reason in both studies. Indeed, adverse effects were mostly mild (gastrointestinal) and in most cases, did not require interruption of treatment.
Overall survival of the drug is useful for assessing long-term adherence in real-world clinical practice. In our study, apremilast survival at week 52 was similar to that of other studies, such as that by Lee et al.6 and Kishimoto et al.,7 with a much higher median survival time compared to other studies published to date (Table 2). This difference may be explained by the inclusion of patients with psoriatic arthritis and the long data-analysis period of our study (5 years).
Review of the Literature on Apremilast Survival Studies in Patients with Psoriasis.
| Vujic I, et al.8 (n=48) | Papadavid E, et al.9 (n=51) | Lee EB, et al.6 (n=84) | Santos-Juanes J, et al.10 (n=48) | Lunder T, et al.11 (n=94) | Kromer C, et al.12*(n=35) | Ohata C, et al.13 (n=50) | Kishimoto M, et al.7(n=138) | Saruwatari, H et al.14 (n=46) | Sbidian E, et al.15 (n=3207) | Del Alcázar E, et al.5 (n=292) | Our study (n=65) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline PASI | 10.7 | 10.8 | - | - | 11.9 | - | 10.1 | - | - | - | 10.7 | 10.06 |
| Drug survival, % (week) | - | 53.4 (52) | 50.6 (143) | 35.41 (143) | 20 (52) | - | 70 (28) | 53.4 (52) | 46.8 (52) | 69 (52) | 54.9 (52) | 50 (52) |
| Median survival time, weeks | 12.50 | ? | 41.29 | 28.57 | - | 65.18 | ? | 64.71 | - | - | - | 152.14 |
-: Not recorded;
?: Not achieved during the study follow-up period.
The principal limitations of our study are its retrospective and observational nature, and a small sample size for each of the psoriasis variants. Strong points include the fact that this is an effectiveness and survival study with a 5-year data-inclusion period and a review of published survival studies.
In conclusion, apremilast is an effective drug for the treatment of psoriasis and psoriatic arthritis in real-world clinical practice. Overall survival in our series is long, probably due to the drug's good safety profile, and it was not affected by the severity of the psoriasis at the start of treatment.