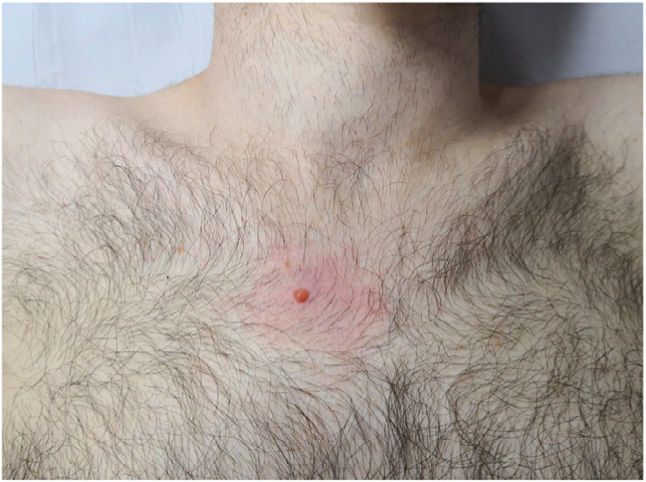
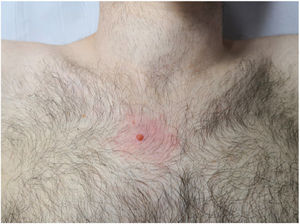
A 25-year-old man presented with a lesion in the right pectoral area that had grown progressively since its appearance 2 years earlier. He denied a personal or family history of skin cancer or other cancers. Physical examination revealed a firm orange papule with a diameter of 5mm and perilesional erythema (Fig. 1). No similar lesions were observed elsewhere.
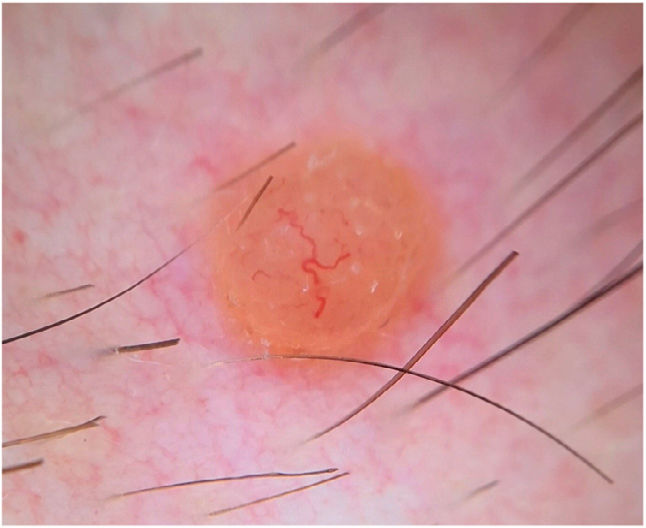
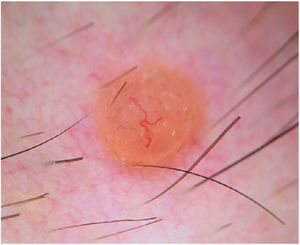
Dermoscopy showed a structureless, homogeneous orange pattern with multiple serpentine vessels distributed throughout the lesion and a branched, larger-caliber telangiectatic vessel crossing the lesion eccentrically (Fig. 2). Histologic and immunohistochemical findings are shown in Fig. 3.
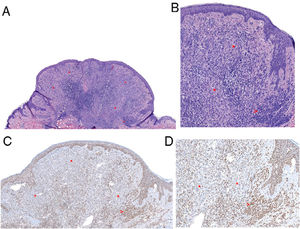
Histologic images. A, Combined polypoid intradermal melanocytic proliferation with a peripheral melanocytic population consistent with a common nevus (arrow) and a second central spitzoid population (asterisks). Note the moderate lymphocytic inflammatory infiltrate (hematoxylin–eosin, original magnification 40). B, Common nevus at the periphery comprising nests of small clonal melanocytes (arrow). Central spitzoid population, with an abundant eosinophilic cytoplasm and discrete nuclear pleomorphism and no evidence of mitosis (hematoxylin–eosin, original magnification 100). C and D, Immunohistochemistry showing loss of BAP1 expression in the spitzoid (asterisks) but not common nevus component (arrow) of the lesion (BAP1, original magnification 100 and 200, respectively).
BAP1-inactivated melanocytic tumor (BIMT).
CommentBIMTs, informally known as bapomas, are melanocytic tumors with a unique genetic profile in that they show inactivation of BRCA1 associated protein 1 (BAP1), a tumor suppressor gene.1,2 Some authors consider BIMTs to be a variant of Spitz nevus, while others consider them to be a separate entity with some overlapping clinical and cytological characteristics.2,3 Although BIMTs are not malignant (there have been no reports of malignant behavior or potential), multiple lesions are a marker of a familial autosomal dominant cancer syndrome caused by a germline inactivating BAP1 mutation.4 Diagnosis of BIMT is important, as the mutation predisposes to several cancers, including pleural and peritoneal mesothelioma, uveal and cutaneous melanoma, nonmelanoma skin cancer, meningioma, lung adenocarcinoma, and renal cell carcinoma.4,5 Some studies have suggested that BIMTs precede other cancers, with a mean age at diagnosis of 30 years.6 Sporadic BIMTs (i.e., tumors without a syndromic association), have also been described.2,6 We did not conduct a germline genetic study, as there was no personal or family history of cancer, and we observed no similar or suspicious skin lesions. The patient was referred for annual clinical follow-up. At the time of writing, 2 years later, he has not developed any other lesions.
Clinically, BIMTs are nonspecific, polymorphous lesions. They generally present as pink or orange papulonodular lesions. The differential diagnosis is broad and includes both melanocytic tumors (intradermal melanocytic nevus, Spitz nevus, melanoma) and nonmelanocytic tumors (neurofibroma, xanthogranuloma, basal cell carcinoma).1,2 The dermoscopic features of BIMTs were recently described2 and can be very useful for aiding diagnosis. Several dermoscopic patterns have been identified: a structureless pink-orange pattern with linear peripheral vessels; a structureless, homogeneous, pink-orange pattern; a structureless pink-orange pattern with radial vessels; a structureless homogeneous pink-orange pattern with atypical eccentric globules; and a network pattern with raised, structureless pink-orange areas.2 The last 2 patterns may be more common in patients with multiple BIMTs and a known germline mutation.2 The globular pattern, by contrast, is uncommon in BIMTs, and appears to be a negative predictor of germline mutations in BAP1, as it has only been described in sporadic BIMTs.2 Dermoscopy in the current case showed a variant of the structureless pink-orange pattern with linear vessels (one of the most common patterns described), with both central and peripheral vessels. The use of contact dermoscopy in the study of dermoscopic features of BIMTs by the International Dermoscopy Society2 may explain why vessels were not seen in the center of the tumors.
Histologically, BIMTs are similar to Spitz nevi, but they do not show epidermal hyperplasia, hypergranulosis, or Kamino bodies.2 They typically show 2 cell populations: nevus cells with a conventional appearance located at the periphery of the lesion and atypical, epithelioid cells with a spitzoid appearance that lack melanin.1,6 Immunohistochemical staining shows clear nuclear loss of BAP1 in this atypical spitzoid population.1,6
In conclusion, BIMTs require a high index of clinical suspicion, but their diagnosis may have important prognostic and follow-up implications. Familiarity with dermoscopic features can help.
Conflicts of InterestThe authors declare that they have no conflicts of interest.