Superficial fungal infections are common in dermatology and are often caused by opportunistic species in the Candida and Malassezia genera. The aim of this study was to analyze changes in the expression of genes coding for enzymes involved in the biosynthesis of glycosaminoglycans (GAGs) chains following the adherence of Candida and Malassezia yeasts to skin cell lines. Gene expression was analyzed using reverse transcriptase–quantitative polymerase chain reaction assays. Interactions between the yeasts and the skin cells induced the following changes in genes involved in the biosynthesis of heparan sulfate and chondroitin sulfate: downregulation of CHPF in keratinocytes and downregulation of EXT1, EXT2, CHSY3, and CHPF in fibroblasts. Adherence to fibroblasts had an even greater effect on GAG biosynthetic enzymes, inducing the downregulation of 13 genes and the upregulation of two (CHST15 and CHST7). Interactions between yeasts and skin cells might affect the binding affinity of GAG chains, possibly changing their ability to function as receptors for pathogens and interfering with a key stage at the start of infection.
Las micosis superficiales son patologías prevalentes en dermatología, causadas frecuentemente por hongos oportunistas de los géneros Candida y Malassezia. El objetivo de este trabajo es analizar, mediante qRT-PCR, la existencia de alteraciones en la expresión génica de las enzimas biosintéticas de las cadenas de glicosaminoglicanos (GAGs) tras la adhesión de dichas levaduras a líneas celulares de piel. La interacción de C. albicans y Malassezia spp. produjo las siguientes modificaciones en genes implicados en la biosíntesis del heparán y condroitín sulfato: la subexpresión de CHPF en los queratinocitos y 4 subexpresiones (EXT1, EXT2, CHSY3 y CHPF) en los fibroblastos. Las enzimas implicadas en la modificación de las cadenas de dichos GAG se ven más alteradas en los fibroblastos, produciendo 13 subexpresiones y 2 sobreexpresiones (CHST15 y CHST7). Como consecuencia, la afinidad de las cadenas de GAGs por sus ligandos puede verse afectada, pudiendo alterar su papel como receptores de microorganismos, paso clave para el inicio de su proceso infeccioso.
Superficial mycoses are prevalent dermatological diseases. The most frequent opportunistic fungi in this type of infection are yeasts of the genera Candida and Malassezia, followed by other primary cutaneous pathogenic filamentous fungi. Development of these superficial skin conditions involves the participation of receptors that allow pathogens to adhere to and colonize the tissue. In addition to helping anchor the fungus to the epithelium, these receptors participate in other aspects of the infectious process, including tissue tropism, induction of the immune response, and tissue invasion.1 Previous studies have demonstrated the role of proteoglycans (PGs), and specifically their glycosaminoglycan (GAG) chains, as receptors in the development of bacterial infections.2 The most important GAGs are heparan sulfate (HS) and chondroitin sulfate (CS).3 Both are composed of a glucuronic acid (GlcA) residue, which is linked to N-acetylglucosamine (GlcNAc) in HS and to N-acetylgalactosamine (GalNAc) in CS.3 Synthesis of HS and CS involves a sequence of events, including polymerization of the chain and its subsequent modification via a series of enzymatic reactions such as N-deacetylation/N-sulfation, epimerization, and/or various O-sulfations.
GAGs are involved in a wide variety of biological and pathological processes: the latter include multiple infectious processes in which alterations in the expression of genes involved in GAG biosynthesis have been described.2,4 In the present study, we investigated whether adherence of C. albicans and Malassezia species to epithelial cells induces changes in the expression of these genes. The objective was to further our knowledge of the role in infectious processes of these fungi, which are capable of causing several diseases under different conditions.
Material and MethodsCell lines and fungal cultures were grown as previously described.5 Cultures of keratinocytes and fibroblasts were grown in 6-well plates, to which 400μL of yeast suspension (corresponding to an A600 of 0.5) and 2mL of Dulbecco's modified Eagle minimal essential medium (DMEM) (Gibco) were added. The plates were then incubated for 90min at 37̊C and 5% CO2. Control wells were treated identically, except only DMEM was added in the last step. After 2 washes with PBS, the culture medium corresponding to each cell line6 was added and the plates were incubated for 16h at 37̊C and 5% CO2. RNA extraction and complementary DNA (cDNA) synthesis were carried out using the RNeasy Mini Kit (Qiagen) and High Capacity cDNA Reverse Transcription Kit (Applied Biosystems), following the manufacturer's instructions. Reverse transcriptase quantitative polymerase chain reaction and data analysis were carried out as previously described,7 using glyceraldehyde 3-phosphate dehydrogenase (G3PDH) as a control gene to normalize expression levels. The oligonucleotide sequence used is described in Supplementary Table 1.
ResultsInteraction of the yeasts with keratinocytes and fibroblasts induced transcriptional alterations in different genes involved in the biosynthesis of HS and CS, depending on the cell line and the microorganism involved.
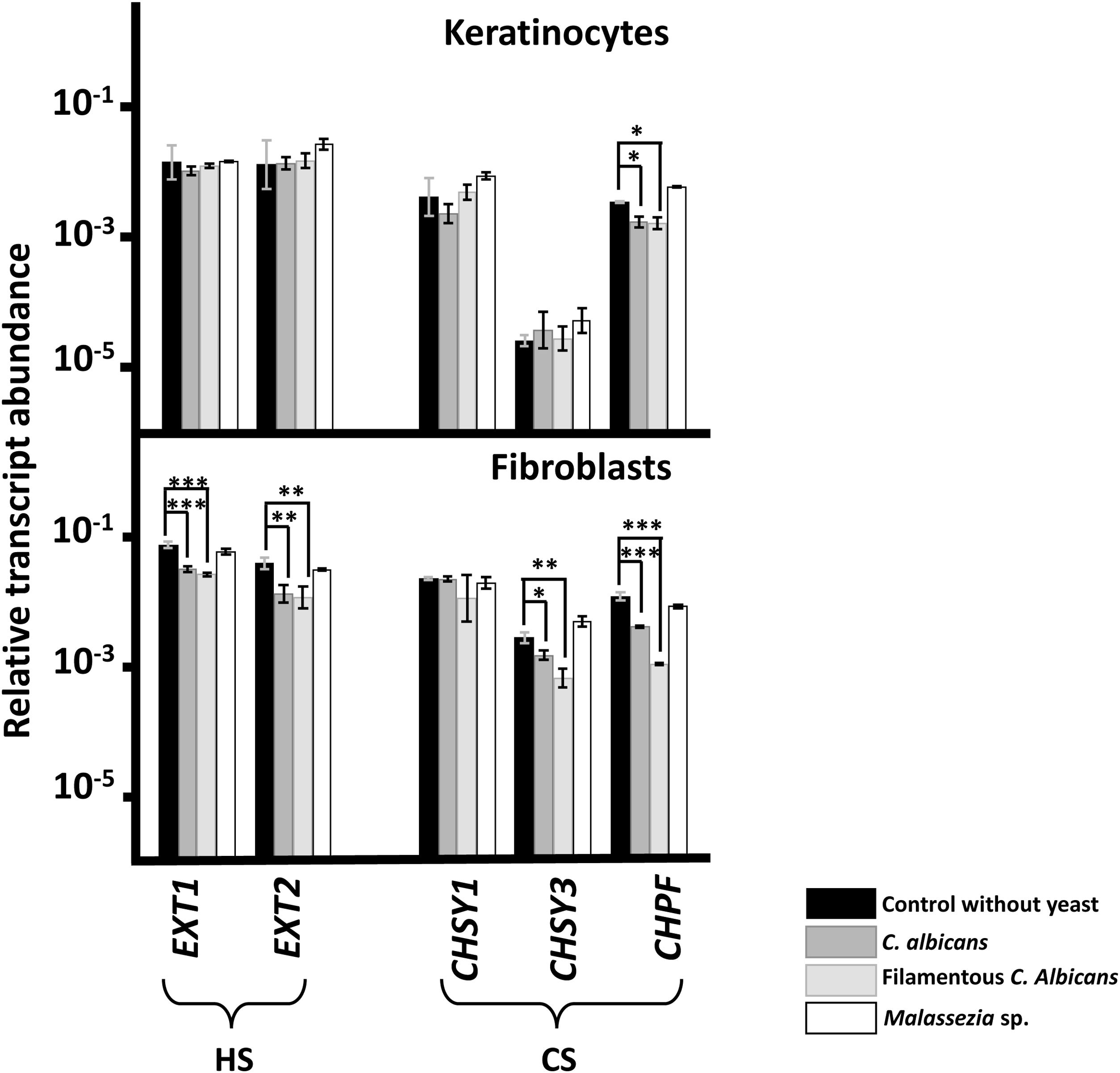
No changes in the transcription of any genes responsible for GAG chain polymerization were observed after adhesion of Malassezia species to either cell line (Fig. 1). By contrast, adherence of C. albicans did induce changes, resulting in reduced expression of CS polymerization factor (CHPF) in keratinocytes and of all genes analyzed, with the exception of CHSY1, in fibroblasts (Fig. 1). Similar results were obtained regardless of the morphology of C. albicans (Fig. 1).
Differential transcription of genes involved in the elongation of heparan sulfate (HS) and chondroitin sulfate (CS) chains in epidermal keratinocytes and dermal fibroblasts after interaction with yeast. Graphs represent the relative abundance of transcripts of genes involved in the polymerization of HS (EXT1 and EXT2) and CS (CHSY1, CHSY3, and CHPF) chains in the absence of yeast (black bars), in the presence of C. albicans (dark gray bars), in the presence of the filamentous form of C. albicans (light gray bars), and in the presence of Malassezia species (white bars).
Data are expressed on a logarithmic scale, and error bars represent the standard deviation. Significant differences are represented as follows: *P<0.05; **P<0.01; ***P<0.001.
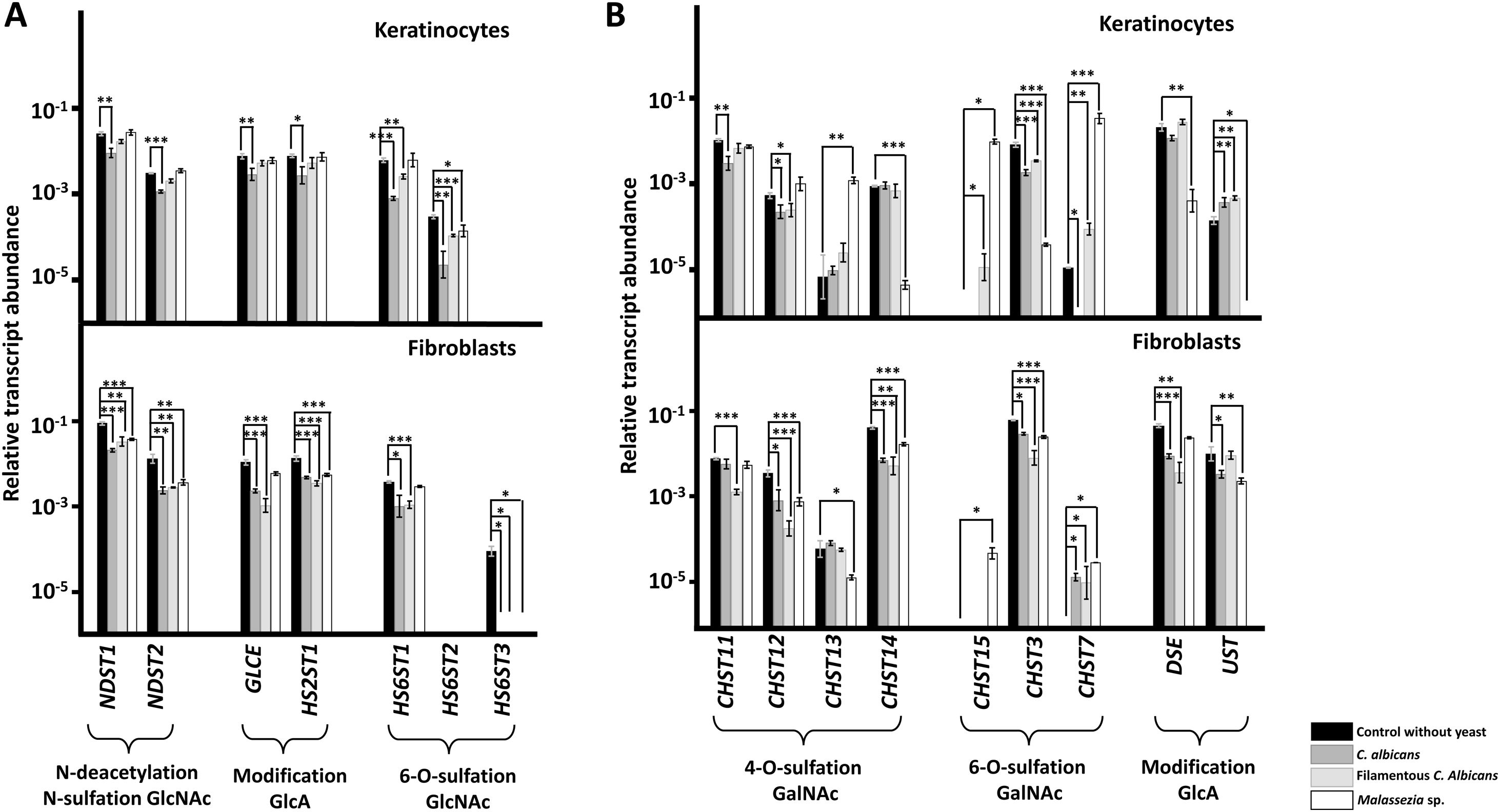
A specific decrease in the expression of genes encoding HS chain-modifying enzymes was observed in the presence of yeast (Fig. 2A). In keratinocytes, the yeast form of C. albicans affected all genes, while the hyphal form only affected those involved in the 6-O-sulfation of GlcNAc. Malassezia species only resulted in reduced expression of the HS6ST2 isoform (Fig. 2A). In fibroblasts, C. albicans, regardless of its cellular form, was associated with reduced expression of all genes, while Malassezia species affected expression of all genes except the gene responsible for epimerization of GlcA (GLCE) and the main isoform responsible for 6-O-sulfation of GlcNAc (HS6ST1) (Fig. 2A).
Differential transcription of genes involved in the modification of heparan sulfate (A) and chondroitin sulfate (B) chains in epidermal keratinocytes and dermal fibroblasts after interaction with yeasts. Graphs represent the relative abundance of transcripts of genes involved in the N-deacetylation/N-sulfation of N-acetylglucosamine (GlcNAc; NDST1-2), epimerization and 2-O-sulfation of glucuronic acid (GlcA; GLCE, HS2ST1), and 6-O-sulfation of GlcNAc (HS6ST1-3), in the absence of yeast (black bars), in the presence of C. albicans (dark gray bars), in the presence of the filamentous form of C. albicans (light gray bars), and in the presence of Malassezia species (white bars).
Data are expressed on a logarithmic scale, and error bars represent the standard deviation. Significant differences are represented as follows: *P<0.05; **P<0.01; ***P<0.001.
Genes encoding CS chain-modifying enzymes exhibited more complex modifications (Fig. 2B). Adhesion to keratinocytes of C. albicans, in either yeast or hyphal form, resulted in reduced expression of CHST12 and CHST3 and increased expression of UST. The yeast form also reduced the expression of CHST11 and CHST7, while the hyphal form increased expression of CHST15 and CHST7 (Fig. 2B). Adhesion of Malassezia species reduced the expression of CHST14, CHST13, DSE, and UST, and increased the expression of CHST13, CHST15, and CHST7 (Fig. 2B). Adherence to fibroblasts of both yeast and hyphal forms of C. albicans reduced the expression of CHST12, CHST14, CHST3, and DSE, and increased the expression of CHST7. Furthermore, adhesion of the yeast form reduced expression of UST, while adhesion of the hyphal form reduced that of CHST11 (Fig. 2B). In fibroblasts, Malassezia species reduced the expression of CHST12, CHST13, CHST14, CHST3, and UST, and increased the expression of CHST15 and CHST7 (Fig. 2B).
DiscussionC. albicans and Malassezia species are microorganisms commonly found in the skin and mucous membranes. Under certain conditions, they can increase in quantity and become true pathogens, triggering a marked immune response. These yeasts can invade and colonize epithelial tissue via several pathways. However, little is known about the initial adhesion of these fungi to the epithelial surface.
In the present study, we observed variations in expression levels of several genes involved in the polymerization and modification of HS and CS chains after interaction with C. albicans and Malassezia species. These alterations were more marked in fibroblasts than keratinocytes. The modifications observed at the transcriptional level point to important changes in the sulfation pattern of the chains, which may be further accentuated by the presence of additional post-transcriptional mechanisms or by the regulation of enzymatic catalysis.8 Consequently, the affinity of GAG chains for certain ligands may be altered, in turn affecting various biological processes, as well as the role of GAGs as receptors for various microorganisms. These modifications may also affect the adherence capacity of other epidermal pathogenic microorganisms, such as Staphylococcus aureus, Streptococcus pyogenes, and Candida species.2,9
The dermal layer, which is less exposed to the environment than the epidermis, differs from the latter in terms of both the receptors expressed and the composition of PGs and GAGs on the cell surface, and therefore would also be expected to differ in its interaction with these microorganisms.9,10 This view is supported by the differential binding of bacteria to different lung cells depending on the GAG species expressed.11 Moreover, this could explain the greater number of alterations detected in the fibroblasts of the dermis: these changes could be caused by the microorganism in order to destabilize the dermal tissue, thereby facilitating invasion.
Yeast adhesion caused greater alterations in enzymes involved in chain modification than those involved in chain polymerization. Expression of genes involved in the modification of HS chains was reduced in both cutaneous layers, albeit to a lesser extent in keratinocytes, in which most changes were observed in the presence of the yeast form of C. albicans. The hyphal form of C. albicans exerted greater effects in fibroblasts, in which the observed alterations were similar to those observed in keratinocytes. The yeast form of C. albicans is normally responsible for the early stages of infection, including initial adhesion and subsequent dissemination, while the formation of hyphae is implicated in the invasion of the underlying tissues.12 This may explain why the former resulted in greater alterations in genes involved in HS biosynthesis in the epidermis. Moreover, the reduced expression of these genes could be associated with the invasive capacity and the immune response. Adhesion of Malassezia species appeared to induce more alterations in the deep layers of the dermis. The role of Malassezia species in skin diseases remains unclear. Through the production of free fatty acids, Malassezia species alters the integrity of the skin, causing irritation and triggering an inflammatory response with consequent release of proinflammatory cytokines, which is associated with multiple pathologies including seborrheic dermatitis and folliculitis.13–15
The alterations described here indicate that GAG chains undergo distinct modifications in sulfation and epimerization depending on the fungus to which they are exposed and the cell type in which they mediate the adhesion and colonization process. This has been previously described for bacteria, whereby different adhesins exhibit affinity for distinct GAG species and even for specific tissues targeted for infection, indicating a certain degree of tropism.7,9 Furthermore, the differential effects of C. albicans, depending on its morphology, may be explained by the differences in cell wall composition.16 Further research is needed to determine the implications on the infectious processes this fungus causes. This knowledge could facilitate the development of new therapies that reduce the incidence of superficial mycoses, such as topical preparations containing GAGs that inhibit adherence of the fungus.
FundingThis study was funded by the 2019 AEDV Investigates (AEDV Investiga) prize, awarded by the Healthy Skin Foundation (Fundación Piel Sana) of the Spanish Academy of Dermatology and Venereology (AEDV).
Conflicts of InterestThe authors declare that they have no conflicts of interest.