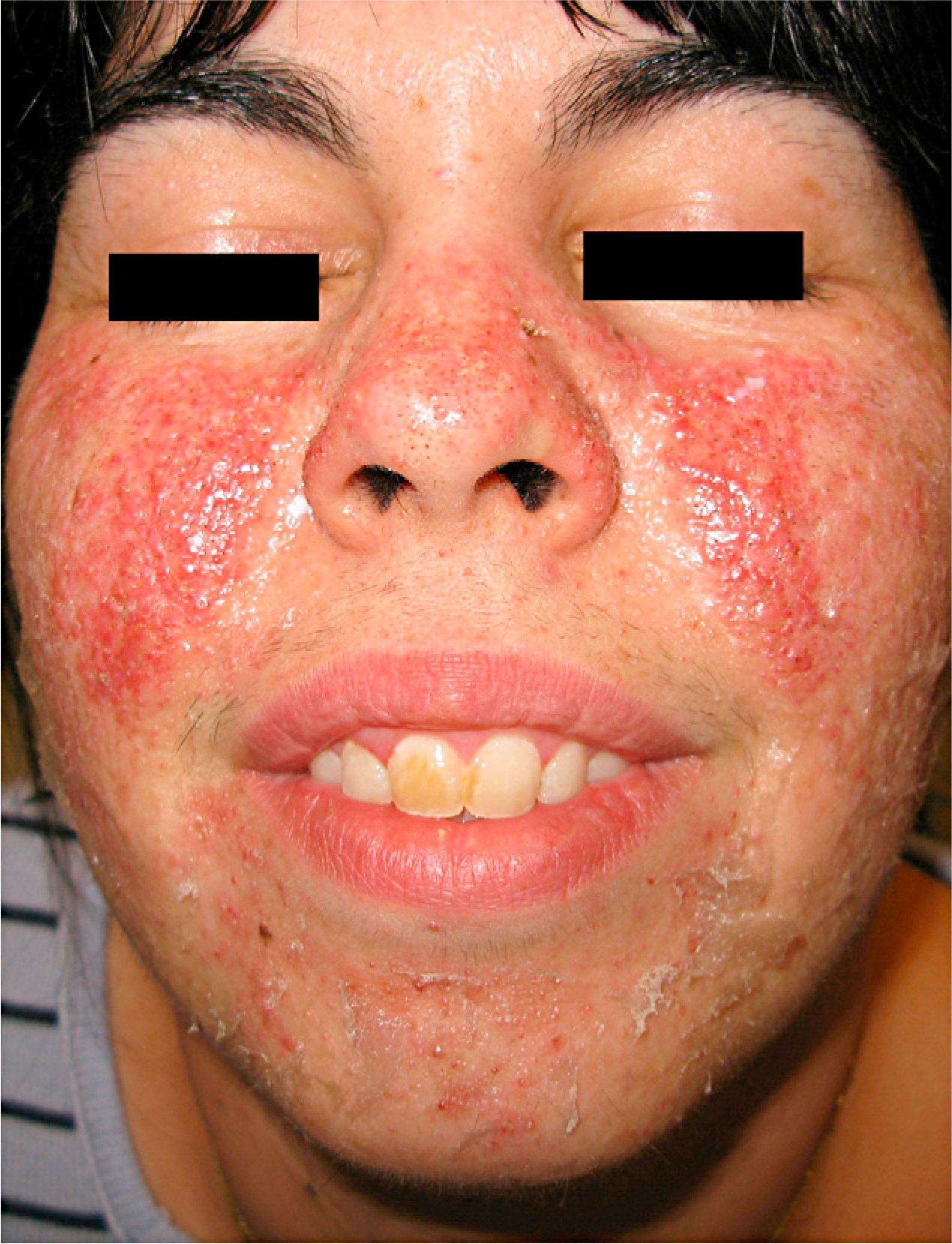
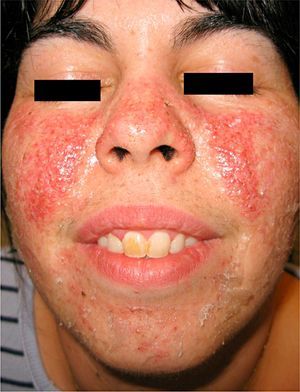
We report the case of a 27-year-old woman with a 10-year history of tuberous sclerosis. Her first visit to the dermatologist revealed several of the cutaneous manifestations characteristic of the disease, namely, disseminated facial angiofibromas (Fig. 1), multiple periungual Koenen tumors on both feet, hypopigmented macules on the trunk, and a shagreen plaque on the back. The patient also had epilepsy and mental retardation. She had no known internal hamartomatous lesions.
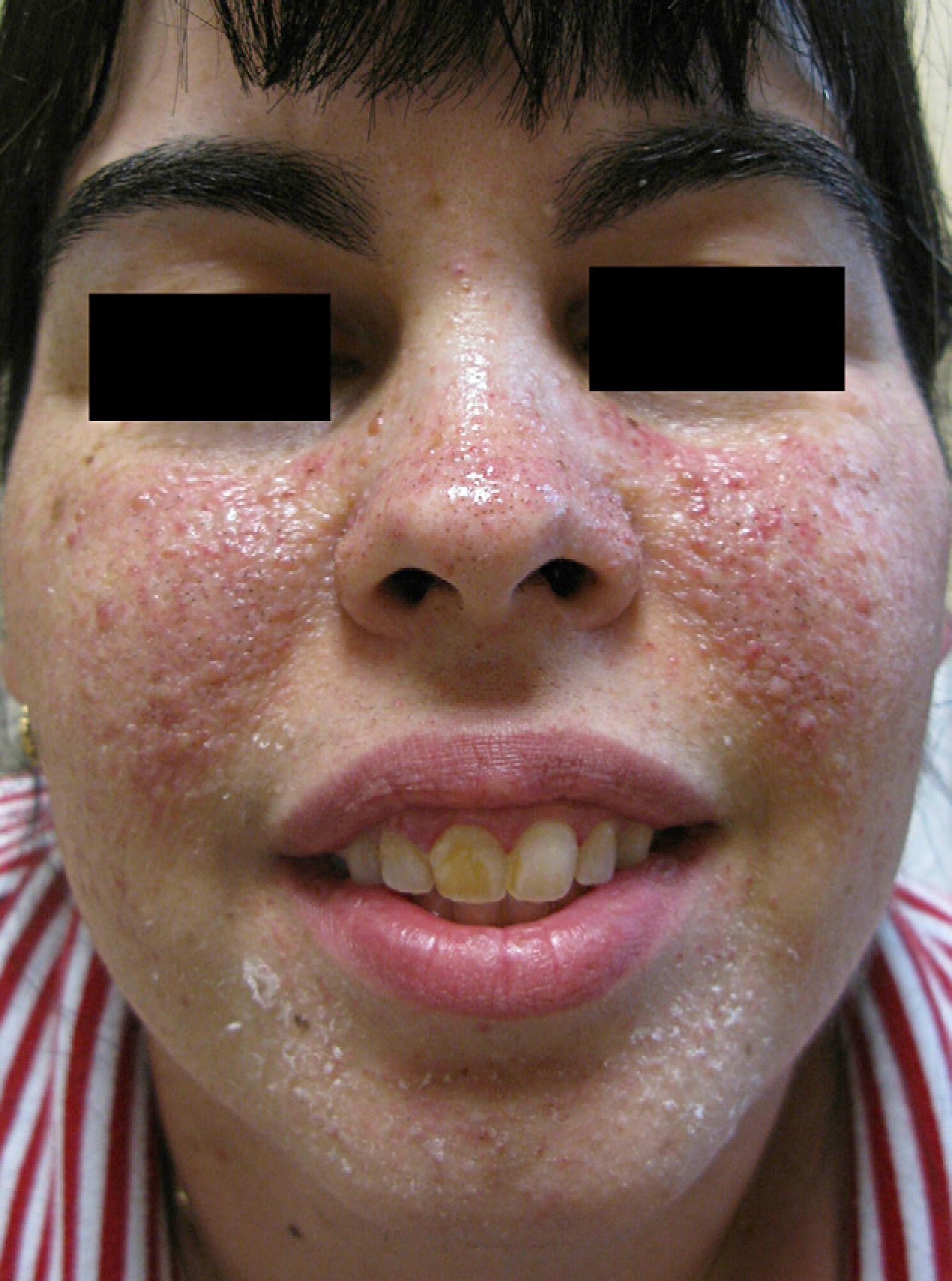
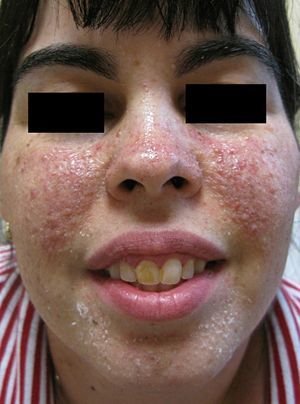
A number of treatments—multiple shave excisions, pulsed-dye laser, electrodessication, and 0.1% topical tacrolimus ointment—had been applied in order to improve her facial appearance and reduce the number of angiofibromas. The response to treatment had been poor, with no appreciable reduction in the number of lesions and persistence of erythema. At that time, pharmacy-prepared topical rapamycin, 1mg/mL, was applied twice daily on the affected areas of both cheeks. A clear clinical improvement was observed after 3 months’ treatment, with a reduction in the number of lesions and in the underlying erythema (Fig. 2).
Tuberous sclerosis is an autosomal-dominant genodermatosis characterized by hamartomas affecting various organs, including the skin and central nervous system. Its pathogenesis is based on abnormalities of the proteins hamartin and tuberin, which are coded on the loci 9p34 and 16p13.3, respectively.1 Symptoms comprise the classic triad of multiple angiofibromas, epilepsy, and mental retardation, although this combination is only seen in 26% of patients.2
Despite its wide clinical variability and variable penetrance,3 facial angiofibromas are found in 83–90% of cases.3,4 These lesions are considered pathognomic and develop mainly on the nasolabial folds, cheeks, chin, scalp, forehead, and ears.4 They usually appear during the first decade of life, stabilize during adolescence, and are lifelong. They are not malignant, although their appearance constitutes a very frequent presenting complaint in these patients.
Treatment can take several forms, including simple excision, cryosurgery, curettage, dermabrasion, carbon dioxide laser, and photodynamic therapy. No single treatment has proven sufficiently effective to control their onset or prevent recurrence.5–7
Rapamycin (sirolimus) is an oral immunosuppressive agent used mainly in kidney transplantation. Its mechanism of action has not been clearly defined, although it is known to interfere with the mTOR protein pathway, which is responsible for cell proliferation and inhibition of apoptosis in patients with tuberous sclerosis.8 The proteins hamartin and tuberin also suppress mTOR pathway activity. These proteins are modified in tuberous sclerosis in such a way that their altered function causes permanent activation of the mTOR pathway, thus leading to the onset of hamartomatous tumors in various regions.9 It has been suggested that the mechanisms by which rapamycin reduces the number and size of tumors in tuberous sclerosis are inhibition of angiogenesis9,10 and of aberrant growth factors,10 although these phenomena have only been verified in extracutaneous hamartomatous lesions (brain, kidney, and lung).
After the failure of the therapeutic approaches adopted to control the facial angiofibromas in our patient, we decided to try topical treatment with rapamycin, an alternative that has been described in 2 previous publications.9,10 Ours is the fourth reported case in which this therapy was administered to control facial angiofibromas. A clear improvement was observed in all 4 patients, with a marked reduction or complete disappearance of the lesions. Facial erythema also improved after only a few months’ treatment. Of the 3 cases reported previously, 2 were treated with rapamycin solution (1mg/mL) and the remaining patient with 0.1% rapamycin ointment. Treatment was administered in 1 or 2 applications per day and, as in our patient, seemed to be well tolerated, with no local or systemic adverse events. The pharmacological basis for the efficacy of rapamycin in treating facial angiofibromas in patients with tuberous sclerosis should be clarified in future studies.
No commercial preparations of topical rapamycin are currently available in the United States or Europe; therefore, the formulation has to be prepared in the pharmacy. In theory, topical administration should be safer than oral medication, although longer follow-up is necessary before these observations can be confirmed. Given the small number of cases described to date, the most suitable presentation of the product has not yet been determined.
In conclusion, we report a new case of multiple facial angiofibromas treated with topical rapamycin in a patient with tuberous sclerosis. This drug could be a valid alternative in this setting, although more cases must be reported in order to verify the long-term safety and efficacy profile of the medication.
Please cite this article as: Valerón-Almazán P, et al. Utilización de solución de rapamicina tópica para el tratamiento de múltiples angiofibromas faciales en una paciente con esclerosis tuberosa. Actas Dermosifiliogr. 2012;103:165–6.