Tuberous sclerosis is an autosomal dominant neurocutaneous disorder caused by mutations in tumor suppressor genes TSC1 (in chromosome 9q34), which encodes hamartin, and TSC2 (in chromosome 16p13.3), which encodes tuberin. Hamartin and tuberin, under normal circumstances, form a complex that inhibits the mammalian target of rapamycin (mTOR), which plays a crucial role in cell-cycle regulation. Mutations in the TSC1 and TSC2 genes lead to defective functioning of these proteins and result in uncontrolled cell proliferation that is characterized by the formation of hamartomas in multiple organs, including the skin and kidneys, and in the central nervous system.1,2 Rapamycin (sirolimus) is an immunosuppressant that inhibits the mTOR pathway. Its only approved indication is prophylaxis of renal transplant rejection. Thanks to its antineoplastic properties, sirolimus inhibits angiogenesis and tumor cell proliferation, and has also been shown to be effective in reducing the number and size of tumors in patients with tuberous sclerosis. Recent publications suggest that topical rapamycin is effective for treating facial angiofibromas3–7 and reducing hypomelanotic macules8 in patients with tuberous sclerosis.
We report a case of a 13-year-old boy who had been clinically diagnosed with tuberous sclerosis at age 4 months based on the presence of typical manifestations of this condition, namely epilepsy, multiple hypomelanotic macules, and facial angiofibromas. Genetic analysis confirmed sporadic tuberous sclerosis caused by a c5043C>G mutation in exon 38 of the TSC2 gene, changing the sequence to the N1681K variant. Magnetic resonance imaging of the brain showed multiple cortical and subcortical tubers, multiple intraventricular and subependymal hamartomas, and bilateral retinal astrocytomas. Abdominal ultrasound revealed the presence in the liver and kidneys of multiple angiomyolipomas, which were asymptomatic at the time of evaluation. Skin manifestations included facial angiofibromas that appeared gradually, multiple hypomelanotic macules, a periungual fibroma, and a shagreen patch on the back.
With the aim of improving the patient's facial appearance by reducing the number of angiofibromas, we obtained informed consent to start treatment with a 0.2% rapamycin ointment formulated by the hospital pharmacy and provided at no cost to the patient. We prescribed once-daily application 5 days a week, for 12 months.
The ointment was applied only to the face; because of the high cost of the drug preparation we did not consider treating the macules on the trunk and limbs.
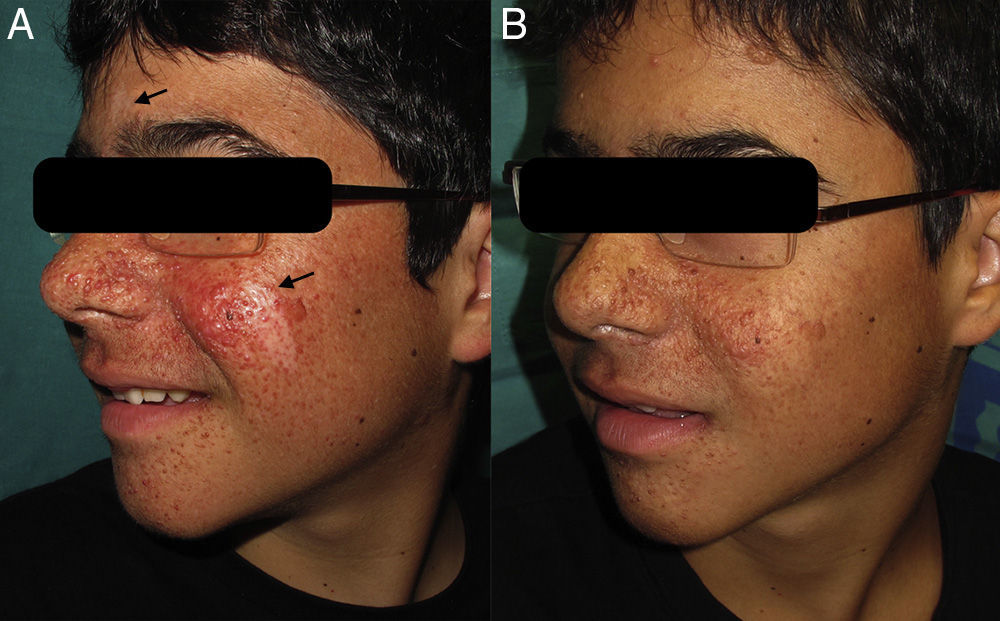
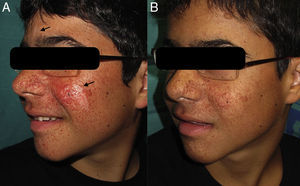
After 2 weeks of treatment we began to observe a reduction in the number of facial angiofibromas, with maximum effect observed at 12 weeks. We also noted considerable improvement in the hypomelanotic macules on the patient's face, with similar responses in the glabella and left cheek (Fig. 1).
Treatment was well tolerated with no local or systemic adverse effects, and plasma rapamycin levels remained below 0.3 ng/mL. We decided to maintain this treatment for a year to prevent the development of new angiofibromas.
Facial angiofibromas are a characteristic feature of tuberous sclerosis. Although benign, they have a considerable psychological impact on patients.
Our case shows that 0.2% topical rapamycin is a safe and effective therapy for both facial angiofibromas and hypomelanotic macules. The high cost of treatment and the lack of an approved indication for topical rapamycin limit the use of this therapeutic agent in daily practice. This is the third case published in the literature describing a marked improvement in hypomelanotic macules, with almost complete remission. The effectiveness of rapamycin against hypomelanotic macules might be explained by the fact that it increases transcription of microphthalmia transcription factor (MITF), which is involved in melanogenic gene expression and in the induction of melanogenesis in melanoma cells.9
In conclusion, the present case further supports the usefulness of topical rapamycin to treat hypomelanotic macules in patients with tuberous sclerosis. However, more patients treated with topical rapamycin are needed in order to establish its safety and long-term effectiveness and to determine the most appropriate treatment regimen.
Please cite this article as: Knöpfel N, Martín-Santiago A, Bauza A, Hervás JA. Rapamicina tópica al 0,2% para el tratamiento de angiofibromas faciales y máculas hipomelanóticas en la esclerosis tuberosa. Actas Dermosifiliogr. 2014;105:802–803.