Retinoids are nonsteroidal hormonal compounds related to retinol. They were discovered more than 50 years ago and have various biologic effects. The oral retinoids currently used in dermatology have different indications and include isotretinoin, acitretin, bexarotene, and alitretinoin. We present the case of a patient who developed thrombocytopenia that was probably induced by acitretin.
The patient was a 78-year-old man with hypertriglyceridemia that had been controlled with fenofibrate for the previous 5 months. He consulted with lesions on the hands that had appeared with a burning sensation 8 months previously. Physical examination revealed delimited hyperkeratotic plaques on the palms and dorsum with nail pitting. He was diagnosed with psoriasis. The results of a complete blood count and biochemistry were normal, and treatment was started with acitretin (Acitretina IFC, 25mg/d). The follow-up analysis performed 6 weeks later revealed a platelet count of 6000/μL (previous, 177 000/μL). The remaining results were normal (hemoglobin, 15.8g/dL; leukocytes, 7900/μL). The patient was asymptomatic, with no bleeding, fever, or other manifestations. A complete blood workup (clotting, biochemistry [kidney profile, liver profile with lactate dehydrogenase], antinuclear antibodies, anti-DNA antibodies, lupus anticoagulant, anticardiolipin antibodies, complement, electrophoresis, immunoglobulins, thyroid hormones, vitamin B12, folic acid, and serology [hepatitis B and C, human immunodeficiency virus]) was performed to rule out possible causes of thrombocytopenia. The results were normal. Abdominal ultrasound revealed fatty liver disease with no splenomegaly. Specialists from the hematology department decided to suspend acitretin as a potential cause, since it had been started only a short time previously. Fenofibrate was maintained, and intravenous immunoglobulin was started (0.4g/kg in 3 doses), as was methylprednisolone (1mg/kg/d, tapering dose). The platelet count returned to normal 4 weeks later (226.000/μL). The skin lesions were treated with topical corticosteroids. The complete blood count remained unaltered 6 months later.
Acitretin is a second-generation monoaromatic retinoid. It is an active metabolite of its precursor, etretinate, and has been marketed since 1997. Acitretin is indicated for severe psoriasis, pustular psoriasis, congenital ichthyosis and ichthyosiform disorders, cutaneous and mucous lichen planus, and severe disorders with dyskeratosis and/or hyperkeratosis.1 It has an antiproliferative effect on psoriatic plaques, reducing thickness, erythema, and desquamation. It also has an anti-inflammatory effect. Given that its pharmacokinetics, effectiveness, and adverse reactions vary from person to person, the dose must be selected on an individual basis, with every attempt made to reach a minimum efficacious dose. Like all retinoids, it is teratogenic, and most adverse effects are dose-dependent and reversible. The most frequent are mucocutaneous effects and lipid and hepatic abnormalities.2 The Summary of Product Characteristics of Neotigason and that of Acitretina IFC do not mention hematologic abnormalities, and a review of the literature reveals few cases. In fact, no cases of thrombocytopenia induced by acitretin have been reported, although 3 cases caused by etretinate (10-50mg/d) have been described in psoriasis patients between 15 days and 2 months after initiation of treatment3–5; the platelet count fell to 2000 in one of the cases and took more than 2 years to return to normal.3 In the remaining cases, values took weeks to return to normal.4,5 The Summary of Product Characteristics of isotretinoin reports anemia, thrombocytopenia, thrombocytosis, and neutropenia as common adverse effects. The literature contains 5 case reports of thrombocytopenia that appeared between a few days and up to 6 months after initiation of isotretinoin.6 Diagnosis of the cases of retinoid-induced thrombocytopenia was based on symptoms once other causes had been ruled out. Other reported hematologic abnormalities include acitretin-induced agranulocytosis (1 case),7 neutropenia (2 cases), and isotretinoin-induced agranulocytosis (2 cases).8,9 Furthermore, there has also been a report of a case of paroxysmal nocturnal hemoglobinuria and another of anemia caused by vitamin B12 and folic acid deficiency induced by isotretinoin. In the case of bexarotene and alitretinoin, the Summary of Product Characteristics reports hematologic abnormalities. Leukopenia, mainly neutropenia, is common with bexarotene, although platelet abnormalities are unusual; thrombocytosis is common with alitretinoin.
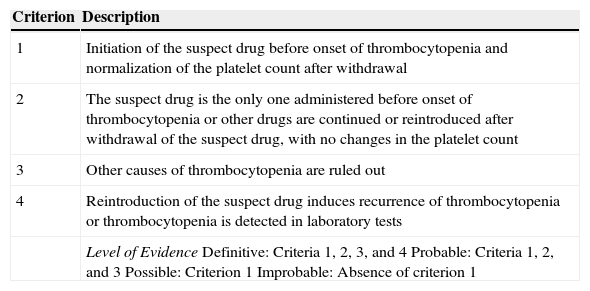
The mechanisms underlying drug-induced thrombocytopenia fall into 2 categories: suppression of platelet production in bone marrow and increased destruction or clearance of platelets in peripheral blood. The first is accompanied by pancytopenia, is usually caused by chemotherapy, and is dose-dependent. The second mechanism is divided into 3 subtypes: the nonimmune subtype, in which the drug has a toxic effect on platelets; the immune subtype, which is caused by drug-specific antibodies that bind to the platelets and is responsible for most cases of drug-induced thrombocytopenia; and the autoimmune subtype, in which antibodies are not drug-dependent. The most frequently involved drugs are quinine and trimethoprim-sulfamethoxazole, although vancomycin, anti-inflammatory drugs, anticonvulsive agents, diuretics, and tuberculostatic drugs also play a role. The incidence of drug-induced thrombocytopenia is around 0.6-1.6 cases/100000 person-years, although this figure may be underestimated. The condition should be suspected mainly in polymedicated hospitalized adults with severe acute thrombocytopenia (generally <10000/μL). Drug-induced thrombocytopenia usually appears 1 to 2 weeks after initiation of therapy, and recurrences are observed early after reintroduction. Patients usually present petechiae, purpura, hematomas, mucosal bleeding, and a risk of internal bleeding (including brain hemorrhage). The condition can also be fatal. Diagnosis is based on clinical findings, depending on the temporal relationship between initiation of drug therapy and onset of thrombocytopenia and after ruling out other causes (infection [mainly viral], vaccination, pregnancy, lymphoproliferative diseases, autoimmune diseases, and idiopathic thrombocytopenic purpura) (Table 1). The ideal approach would be to demonstrate the presence of specific antiplatelet antibodies; however, the relevant tests are not usually available and are not performed as part of daily clinical practice. The platelet count recovers 1 to 2 weeks after the culprit drug has been withdrawn; in severe cases, corticosteroids, immunoglobulins, and even platelet transfusions are necessary. In patients with drug-induced thrombocytopenia, corticosteroids can be withdrawn quickly after the platelet count recovers; in idiopathic thrombocytopenic purpura, however, the course of treatment is longer.10 In the case we report, thrombocytopenia resolved quickly after withdrawal of acitretin and treatment with corticosteroids and immunoglobulins. Given the clear temporal relationship and clinical course after withdrawal of acitretin, we decided not to readminister the potential culprit drug to confirm the diagnosis.
Criteria for the Diagnosis of Drug-Induced Thrombocytopenia.
| Criterion | Description |
|---|---|
| 1 | Initiation of the suspect drug before onset of thrombocytopenia and normalization of the platelet count after withdrawal |
| 2 | The suspect drug is the only one administered before onset of thrombocytopenia or other drugs are continued or reintroduced after withdrawal of the suspect drug, with no changes in the platelet count |
| 3 | Other causes of thrombocytopenia are ruled out |
| 4 | Reintroduction of the suspect drug induces recurrence of thrombocytopenia or thrombocytopenia is detected in laboratory tests |
| Level of EvidenceDefinitive: Criteria 1, 2, 3, and 4Probable: Criteria 1, 2, and 3Possible: Criterion 1Improbable: Absence of criterion 1 |
Source: Chong et al.10
Please cite this article as: García-Arpa M, López-Nieto M, Santiago Sánchez-Mateos JL, Sánchez-Caminero MP. Trombocitopenia en probable relación con acitretina. 2015;106:692–693.