Experience in the use of apremilast in clinical practice complements the information available from pivotal clinical trials.
Materials and methodsFollowing a review of the literature, a panel of dermatologists with expertise in the management of psoriasis considered 5 scenarios in which the evidence supporting the use of apremilast to treat moderate psoriasis is insufficient or controversial. These scenarios were then assessed using a Delphi questionnaire.
ResultsConsensus was reached on 96 (67%) of the 143 items (positive in 85 and negative in 11). The therapeutic goal for apremilast should be based on 4 outcomes: clinical response, symptoms, quality of life, and patient satisfaction. The scenario in which the use of apremilast was considered to have the greatest possibility of success was in patients with stable moderate psoriasis. Most of the clinicians considered apremilast to be an appropriate treatment when conventional therapies fail or are contraindicated, preferably before the prescription of biologic therapy. Consensus was reached that apremilast is an appropriate treatment for psoriasis in difficult locations, such as the scalp or the palms and soles. It was also agreed that apremilast requires less prescreening and monitoring than other conventional and biologic systemic therapies.
ConclusionsApremilast could be a treatment option for patients with a different profile to that of clinical trial participants. The limitations of this proposal are the absence of consensus on the definition of moderate psoriasis, the lack of real-world evidence on the use of apremilast, and certain aspects related to tolerability.
El manejo de apremilast en la práctica clínica complementa la información procedente de los ensayos clínicos pivotales.
Material y métodoTras una revisión de la literatura se consideraron, por parte de un panel de dermatólogos expertos en el manejo de la psoriasis, 5 áreas donde la evidencia sobre el uso de apremilast en psoriasis moderada era insuficiente o controvertida que fueron evaluadas a través de un cuestionario diseñado según la metodología Delphi.
ResultadosSe alcanzó consenso en 96 de los 143 ítems planteados (67%) (85 en el acuerdo y 11 en el desacuerdo). El objetivo terapéutico con apremilast debería ponderarse entre la respuesta clínica, la sintomatología asociada, la calidad de vida y la satisfacción del paciente. El perfil en el que el uso de apremilast se considera con mayores posibilidades de éxito sería el de un paciente con psoriasis moderada estable. La mayoría de los clínicos consideraron que apremilast es adecuado para pacientes en los que hayan fracasado o estén contraindicados los tratamientos convencionales, preferentemente de forma previa a la indicación de terapia biológica. Hubo consenso en reconocer apremilast como una opción terapéutica adecuada para el tratamiento en localizaciones difíciles como la psoriasis palmoplantar y del cuero cabelludo. La necesidad de cribado, así como de su monitorización durante el seguimiento, se consideró menor que la de otros tratamientos sistémicos, convencionales y biológicos.
ConclusionesApremilast podría representar una opción terapéutica en un perfil de pacientes distinto al presentado en los ensayos clínicos. La ausencia de un consenso sobre la definición de psoriasis moderada, la escasa evidencia acerca del fármaco en vida real, así como algunos aspectos relacionados con la tolerabilidad representan limitaciones a estas propuestas.
Apremilast is an oral phosphodiesterase-4 inhibitor with immunomodulatory activity that partially blocks the expression of proinflammatory cytokines and induces the expression of anti-inflammatory cytokines with a pathogenic role in psoriasis. It is indicated for the treatment of moderate to severe psoriasis1 and psoriatic arthritis and has acceptable short- and long-term safety and tolerability profiles. Nevertheless, because the patients studied in the pivotal clinical trials of apremilast had a very specific profile, similar to that of candidates for biologic therapy,2,3 little is known about real-world usage, such as the identification of candidates for treatment or the correct positioning of the drug in relation to conventional and biologic therapies. Consensus-based recommendations based on expert opinions and developed using rigorous, validated methodologies serve as useful guidelines for such situations and provide experts with valuable information on the use of apremilast in everyday clinical practice.4
To complement the existing evidence on the use of apremilast, a group of experts came together to evaluate clinical scenarios and aspects of treatment for which the information in the literature was considered to be lacking.
Material and MethodsExpert PanelThe expert panel was formed by a national coordinator (J.M.C.) and 5 regional coordinators (R.R., I.B., M.G.B., M.A., P.H.). The regional members proposed and discussed different clinical scenarios associated with the use of apremilast for which they considered it would be interesting to develop recommendations in light of the current lack of evidence or consensus. Five scenarios were chosen: i) effectiveness of apremilast in moderate psoriasis as an option prior to biologic therapy in this setting, ii) effectiveness in special locations: nails, scalp, and palms/soles; iii) effectiveness in psoriasis and psoriatic arthritis, iv) prescreening and management of comorbidities in psoriasis, and v) safety issues.
Literature SearchA literature search was performed in the MEDLINE and Cochrane Library databases to identify issues associated with the use of apremilast for which existing evidence was considered to be insufficient to answer questions that arise in routine clinical practice. A separate search strategy, complemented by a hand search, was designed for each scenario. For the evaluation of the different scenarios, priority was given to data from randomized clinical trials and real-world studies, although recent consensus documents, recommendation articles, and clinical guidelines were also included. The literature search was performed in February 2018 and targeted articles originally published in English or Spanish. No time restrictions were placed on clinical trials or real-world studies (Table 1). When this article was finished, additional searches of the same databases were performed to identify new information on the real-world use of apremilast (literature search updated in May 2019).
Literature Search Strategy.
| The search strategies used for each scenario are detailed below: i) Scenario #1: ''(Apremilast OR otezla) AND (psoriasis OR moderate psoriasis OR severe to moderate psoriasis) AND efficacy NOT psoriatic arthritis'' (45 references/12 clinical trials); ii) Scenario #2: ''(Apremilast OR otezla) AND (psoriasis OR moderate psoriasis OR severe to moderate psoriasis) AND efficacy AND (scalp OR nail OR palmoplantar OR difficult to treat) NOT psoriatic arthritis'' (9 references/2 clinical trials); iii) Scenario #3: ''(Apremilast OR otezla) AND (psoriasis OR moderate psoriasis OR severe to moderate psoriasis) AND psoriatic arthritis AND efficacy'' (214 references/2 clinical trials); iv) Scenario #4: ''psoriasis AND comorbid* AND (screening OR management) NOT psoriatic arthritis'' (513 references/8 clinical trials); v) Scenario #5: ''(Apremilast OR otezla) AND (psoriasis OR moderate psoriasis OR severe to moderate psoriasis) AND safety NOT psoriatic arthritis'' (27 references/11 clinical trials) |
Appendix B Annex SI. See supplementary material.5–7
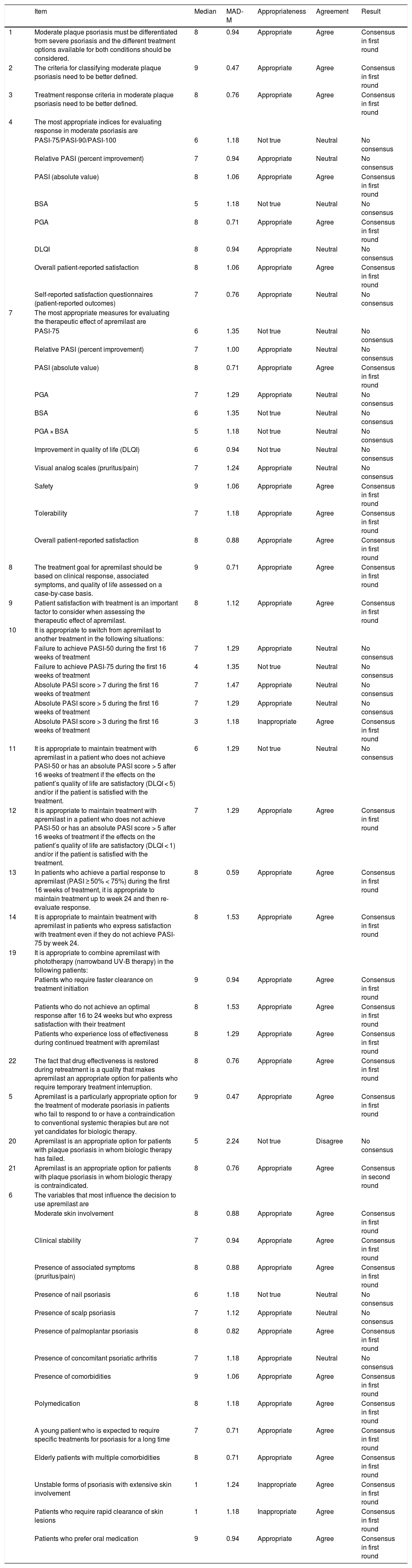
ResultsEffectiveness of Apremilast in Moderate Psoriasis: Use Before Biologic Therapy in Patients With Moderate PsoriasisPositive consensus was reached for 30 (55.6%) of the 54 items (24 in the first round and 6 in the second round) on the use of apremilast before biologic therapy in patients with moderate psoriasis. Negative consensus was achieved for 3 items (5.6%): 2 in the first round and 1 in the second. Absolute Psoriasis and Area Severity Index (PASI) scores and overall patient-reported satisfaction were considered to be the most appropriate variables for evaluating apremilast in clinical practice. The panel agreed that it was appropriate to maintain apremilast up to 24 weeks in patients with a partial response at week 16 (< 50% reduction in PASI or absolute PASI score > 5), as long as they were satisfied with the treatment and had not experienced significant impairment in quality of life (Dermatology Life Quality Index [DLQI] score < 1). It was even considered justifiable to maintain apremilast beyond week 24 in patients who had not achieved PASI-75 if they were satisfied with their treatment.
Apremilast was considered to be an appropriate treatment for patients with moderate psoriasis when conventional systemic treatments fail or are contraindicated and for patients who are not yet candidates for biologic therapy or who do not respond to or have a contraindication to biologic agents. The following factors were identified as having the greatest influence on the decision to prescribe apremilast: i) moderate skin involvement, ii) clinical stability, iii) associated symptoms, iv) presence of palmoplantar psoriasis, v) presence of comorbidities, vi) polymedication, vii) young patients expected to need treatment for a long time, and viii) patients with injection phobia. By contrast, apremilast was not considered appropriate for patients with extensive, unstable psoriasis or for patients requiring rapid clearance.
The combined use of apremilast and phototherapy (particularly narrowband UV-B therapy) was considered appropriate in the following circumstances: i) as a treatment initiation strategy in patients requiring rapid clearance, ii) in patients who fail to achieve an optimal response after 16 to 24 weeks but who are satisfied with their treatment, and iii) in patients who start to show loss of effectiveness (Table 2).
Effectiveness of Apremilast in Moderate Psoriasis: Use Before Biologic Therapy in Patients With Moderate Disease.
| Item | Median | MAD-M | Appropriateness | Agreement | Result | |
|---|---|---|---|---|---|---|
| 1 | Moderate plaque psoriasis must be differentiated from severe psoriasis and the different treatment options available for both conditions should be considered. | 8 | 0.94 | Appropriate | Agree | Consensus in first round |
| 2 | The criteria for classifying moderate plaque psoriasis need to be better defined. | 9 | 0.47 | Appropriate | Agree | Consensus in first round |
| 3 | Treatment response criteria in moderate plaque psoriasis need to be better defined. | 8 | 0.76 | Appropriate | Agree | Consensus in first round |
| 4 | The most appropriate indices for evaluating response in moderate psoriasis are | |||||
| PASI-75/PASI-90/PASI-100 | 6 | 1.18 | Not true | Neutral | No consensus | |
| Relative PASI (percent improvement) | 7 | 0.94 | Appropriate | Neutral | No consensus | |
| PASI (absolute value) | 8 | 1.06 | Appropriate | Agree | Consensus in first round | |
| BSA | 5 | 1.18 | Not true | Neutral | No consensus | |
| PGA | 8 | 0.71 | Appropriate | Agree | Consensus in first round | |
| DLQI | 8 | 0.94 | Appropriate | Neutral | No consensus | |
| Overall patient-reported satisfaction | 8 | 1.06 | Appropriate | Agree | Consensus in first round | |
| Self-reported satisfaction questionnaires (patient-reported outcomes) | 7 | 0.76 | Appropriate | Neutral | No consensus | |
| 7 | The most appropriate measures for evaluating the therapeutic effect of apremilast are | |||||
| PASI-75 | 6 | 1.35 | Not true | Neutral | No consensus | |
| Relative PASI (percent improvement) | 7 | 1.00 | Appropriate | Neutral | No consensus | |
| PASI (absolute value) | 8 | 0.71 | Appropriate | Agree | Consensus in first round | |
| PGA | 7 | 1.29 | Appropriate | Neutral | No consensus | |
| BSA | 6 | 1.35 | Not true | Neutral | No consensus | |
| PGA × BSA | 5 | 1.18 | Not true | Neutral | No consensus | |
| Improvement in quality of life (DLQI) | 6 | 0.94 | Not true | Neutral | No consensus | |
| Visual analog scales (pruritus/pain) | 7 | 1.24 | Appropriate | Neutral | No consensus | |
| Safety | 9 | 1.06 | Appropriate | Agree | Consensus in first round | |
| Tolerability | 7 | 1.18 | Appropriate | Agree | Consensus in first round | |
| Overall patient-reported satisfaction | 8 | 0.88 | Appropriate | Agree | Consensus in first round | |
| 8 | The treatment goal for apremilast should be based on clinical response, associated symptoms, and quality of life assessed on a case-by-case basis. | 9 | 0.71 | Appropriate | Agree | Consensus in first round |
| 9 | Patient satisfaction with treatment is an important factor to consider when assessing the therapeutic effect of apremilast. | 8 | 1.12 | Appropriate | Agree | Consensus in first round |
| 10 | It is appropriate to switch from apremilast to another treatment in the following situations: | |||||
| Failure to achieve PASI-50 during the first 16 weeks of treatment | 7 | 1.29 | Appropriate | Neutral | No consensus | |
| Failure to achieve PASI-75 during the first 16 weeks of treatment | 4 | 1.35 | Not true | Neutral | No consensus | |
| Absolute PASI score > 7 during the first 16 weeks of treatment | 7 | 1.47 | Appropriate | Neutral | No consensus | |
| Absolute PASI score > 5 during the first 16 weeks of treatment | 7 | 1.29 | Appropriate | Neutral | No consensus | |
| Absolute PASI score > 3 during the first 16 weeks of treatment | 3 | 1.18 | Inappropriate | Agree | Consensus in first round | |
| 11 | It is appropriate to maintain treatment with apremilast in a patient who does not achieve PASI-50 or has an absolute PASI score > 5 after 16 weeks of treatment if the effects on the patient’s quality of life are satisfactory (DLQI < 5) and/or if the patient is satisfied with the treatment. | 6 | 1.29 | Not true | Neutral | No consensus |
| 12 | It is appropriate to maintain treatment with apremilast in a patient who does not achieve PASI-50 or has an absolute PASI score > 5 after 16 weeks of treatment if the effects on the patient’s quality of life are satisfactory (DLQI < 1) and/or if the patient is satisfied with the treatment. | 7 | 1.29 | Appropriate | Agree | Consensus in first round |
| 13 | In patients who achieve a partial response to apremilast (PASI ≥ 50% < 75%) during the first 16 weeks of treatment, it is appropriate to maintain treatment up to week 24 and then re-evaluate response. | 8 | 0.59 | Appropriate | Agree | Consensus in first round |
| 14 | It is appropriate to maintain treatment with apremilast in patients who express satisfaction with treatment even if they do not achieve PASI-75 by week 24. | 8 | 1.53 | Appropriate | Agree | Consensus in first round |
| 19 | It is appropriate to combine apremilast with phototherapy (narrowband UV-B therapy) in the following patients: | |||||
| Patients who require faster clearance on treatment initiation | 9 | 0.94 | Appropriate | Agree | Consensus in first round | |
| Patients who do not achieve an optimal response after 16 to 24 weeks but who express satisfaction with their treatment | 8 | 1.53 | Appropriate | Agree | Consensus in first round | |
| Patients who experience loss of effectiveness during continued treatment with apremilast | 8 | 1.29 | Appropriate | Agree | Consensus in first round | |
| 22 | The fact that drug effectiveness is restored during retreatment is a quality that makes apremilast an appropriate option for patients who require temporary treatment interruption. | 8 | 0.76 | Appropriate | Agree | Consensus in first round |
| 5 | Apremilast is a particularly appropriate option for the treatment of moderate psoriasis in patients who fail to respond to or have a contraindication to conventional systemic therapies but are not yet candidates for biologic therapy. | 9 | 0.47 | Appropriate | Agree | Consensus in first round |
| 20 | Apremilast is an appropriate option for patients with plaque psoriasis in whom biologic therapy has failed. | 5 | 2.24 | Not true | Disagree | No consensus |
| 21 | Apremilast is an appropriate option for patients with plaque psoriasis in whom biologic therapy is contraindicated. | 8 | 0.76 | Appropriate | Agree | Consensus in second round |
| 6 | The variables that most influence the decision to use apremilast are | |||||
| Moderate skin involvement | 8 | 0.88 | Appropriate | Agree | Consensus in first round | |
| Clinical stability | 7 | 0.94 | Appropriate | Agree | Consensus in first round | |
| Presence of associated symptoms (pruritus/pain) | 8 | 0.88 | Appropriate | Agree | Consensus in first round | |
| Presence of nail psoriasis | 6 | 1.18 | Not true | Neutral | No consensus | |
| Presence of scalp psoriasis | 7 | 1.12 | Appropriate | Neutral | No consensus | |
| Presence of palmoplantar psoriasis | 8 | 0.82 | Appropriate | Agree | Consensus in first round | |
| Presence of concomitant psoriatic arthritis | 7 | 1.18 | Appropriate | Neutral | No consensus | |
| Presence of comorbidities | 9 | 1.06 | Appropriate | Agree | Consensus in first round | |
| Polymedication | 8 | 1.18 | Appropriate | Agree | Consensus in first round | |
| A young patient who is expected to require specific treatments for psoriasis for a long time | 7 | 0.71 | Appropriate | Agree | Consensus in first round | |
| Elderly patients with multiple comorbidities | 8 | 0.71 | Appropriate | Agree | Consensus in first round | |
| Unstable forms of psoriasis with extensive skin involvement | 1 | 1.24 | Inappropriate | Agree | Consensus in first round | |
| Patients who require rapid clearance of skin lesions | 1 | 1.18 | Inappropriate | Agree | Consensus in first round | |
| Patients who prefer oral medication | 9 | 0.94 | Appropriate | Agree | Consensus in first round |
Abbreviations: BSA, body surface area affected; DLQI, Dermatology Life Quality Index; MAD-M, mean absolute deviation from the median; PASI, Psoriasis Area Severity Index; PASI-75/90/100, reduction of ≥ 75%/≥ 90%/100% in PASI; PGA, physican global assessment.
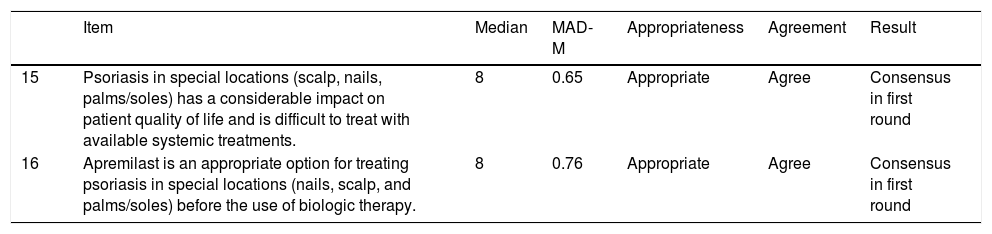
The expert panel agreed in the first round that apremilast was an appropriate treatment for patients with psoriasis affecting special locations, such as the nails, the scalp, and the palms and soles (Table 3).
Effectiveness of Apremilast in Special Locations: Nails, Scalp, and Palms and Soles.
| Item | Median | MAD-M | Appropriateness | Agreement | Result | |
|---|---|---|---|---|---|---|
| 15 | Psoriasis in special locations (scalp, nails, palms/soles) has a considerable impact on patient quality of life and is difficult to treat with available systemic treatments. | 8 | 0.65 | Appropriate | Agree | Consensus in first round |
| 16 | Apremilast is an appropriate option for treating psoriasis in special locations (nails, scalp, and palms/soles) before the use of biologic therapy. | 8 | 0.76 | Appropriate | Agree | Consensus in first round |
Abbreviation: MAD-M, mean absolute deviation from the median.
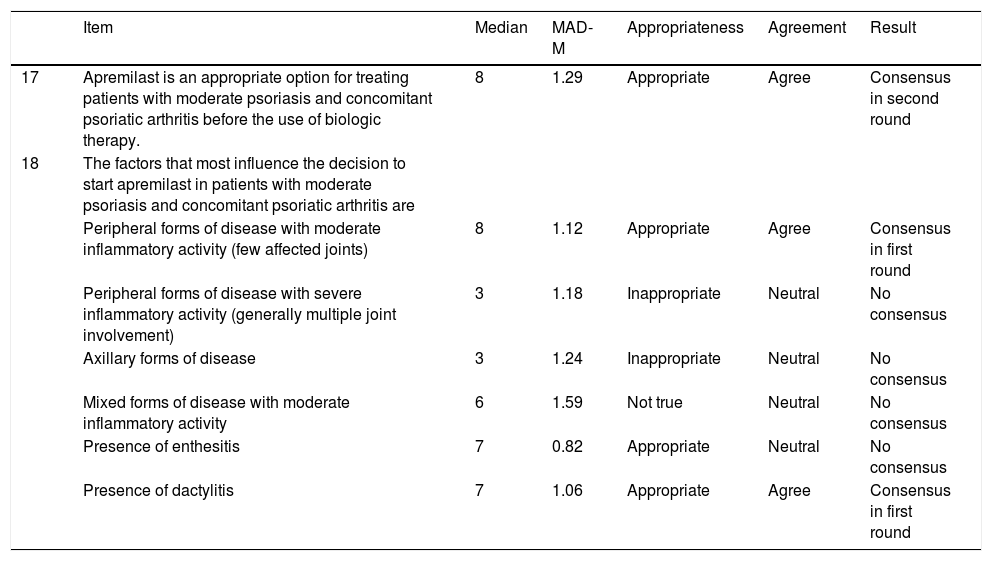
Positive consensus was reached for 3 (42.9%) of the 7 items (2 in the first round and 1 in the second) on the use of psoriasis in patients with concomitant psoriasis and psoriatic arthritis. It was agreed that it was appropriate to use apremilast before biologic therapy in patients with moderate to severe psoriasis and concomitant psoriatic arthritis who have peripheral disease with moderate inflammatory activity (few joints affected) or dactylitis (Table 4).
Effectiveness of Apremilast in Patients With Concomitant Psoriasis and Psoriatic Arthritis.
| Item | Median | MAD-M | Appropriateness | Agreement | Result | |
|---|---|---|---|---|---|---|
| 17 | Apremilast is an appropriate option for treating patients with moderate psoriasis and concomitant psoriatic arthritis before the use of biologic therapy. | 8 | 1.29 | Appropriate | Agree | Consensus in second round |
| 18 | The factors that most influence the decision to start apremilast in patients with moderate psoriasis and concomitant psoriatic arthritis are | |||||
| Peripheral forms of disease with moderate inflammatory activity (few affected joints) | 8 | 1.12 | Appropriate | Agree | Consensus in first round | |
| Peripheral forms of disease with severe inflammatory activity (generally multiple joint involvement) | 3 | 1.18 | Inappropriate | Neutral | No consensus | |
| Axillary forms of disease | 3 | 1.24 | Inappropriate | Neutral | No consensus | |
| Mixed forms of disease with moderate inflammatory activity | 6 | 1.59 | Not true | Neutral | No consensus | |
| Presence of enthesitis | 7 | 0.82 | Appropriate | Neutral | No consensus | |
| Presence of dactylitis | 7 | 1.06 | Appropriate | Agree | Consensus in first round |
Abbreviation: MAD-M, mean absolute deviation from the median.
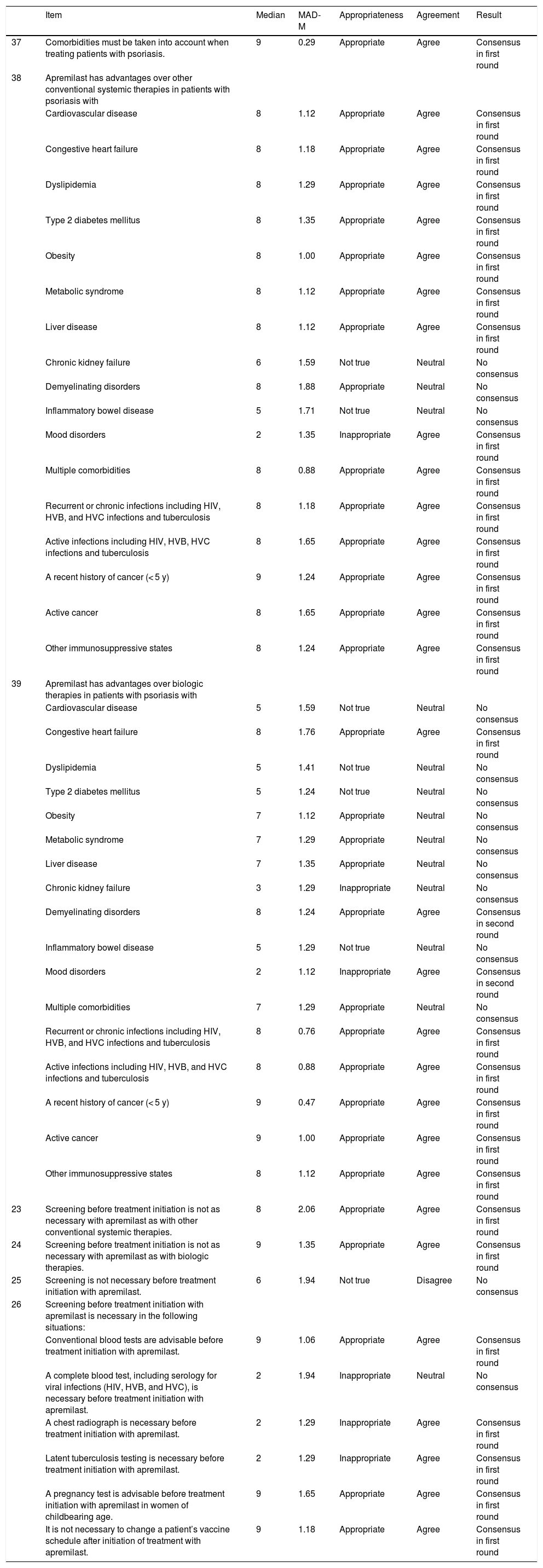
Positive consensus was reached for 26 (59.1%) of the 44 items (20 in the first round and 6 in the second round) in the section on apremilast and comorbidities in psoriasis. Negative consensus was reached for 4 items (9.1%): 2 in the first round and 1 in the second. It was agreed that apremilast offered advantages over other conventional systemic treatments and biologic therapies in terms of its impact on comorbidities in patients with recurrent, chronic, or active infections, a recent history of cancer (< 5 years), active cancer, other immunosuppressive states, or metabolic disorders. The panel also agreed that apremilast was safer than biologic agents for patients with congestive heart disease or demyelinating disorders. By contrast, apremilast was not considered to be an appropriate treatment for patients with mood disorders (Table 5).
Prescreening and Management of Comorbidities in Psoriasis.
| Item | Median | MAD-M | Appropriateness | Agreement | Result | |
|---|---|---|---|---|---|---|
| 37 | Comorbidities must be taken into account when treating patients with psoriasis. | 9 | 0.29 | Appropriate | Agree | Consensus in first round |
| 38 | Apremilast has advantages over other conventional systemic therapies in patients with psoriasis with | |||||
| Cardiovascular disease | 8 | 1.12 | Appropriate | Agree | Consensus in first round | |
| Congestive heart failure | 8 | 1.18 | Appropriate | Agree | Consensus in first round | |
| Dyslipidemia | 8 | 1.29 | Appropriate | Agree | Consensus in first round | |
| Type 2 diabetes mellitus | 8 | 1.35 | Appropriate | Agree | Consensus in first round | |
| Obesity | 8 | 1.00 | Appropriate | Agree | Consensus in first round | |
| Metabolic syndrome | 8 | 1.12 | Appropriate | Agree | Consensus in first round | |
| Liver disease | 8 | 1.12 | Appropriate | Agree | Consensus in first round | |
| Chronic kidney failure | 6 | 1.59 | Not true | Neutral | No consensus | |
| Demyelinating disorders | 8 | 1.88 | Appropriate | Neutral | No consensus | |
| Inflammatory bowel disease | 5 | 1.71 | Not true | Neutral | No consensus | |
| Mood disorders | 2 | 1.35 | Inappropriate | Agree | Consensus in first round | |
| Multiple comorbidities | 8 | 0.88 | Appropriate | Agree | Consensus in first round | |
| Recurrent or chronic infections including HIV, HVB, and HVC infections and tuberculosis | 8 | 1.18 | Appropriate | Agree | Consensus in first round | |
| Active infections including HIV, HVB, HVC infections and tuberculosis | 8 | 1.65 | Appropriate | Agree | Consensus in first round | |
| A recent history of cancer (< 5 y) | 9 | 1.24 | Appropriate | Agree | Consensus in first round | |
| Active cancer | 8 | 1.65 | Appropriate | Agree | Consensus in first round | |
| Other immunosuppressive states | 8 | 1.24 | Appropriate | Agree | Consensus in first round | |
| 39 | Apremilast has advantages over biologic therapies in patients with psoriasis with | |||||
| Cardiovascular disease | 5 | 1.59 | Not true | Neutral | No consensus | |
| Congestive heart failure | 8 | 1.76 | Appropriate | Agree | Consensus in first round | |
| Dyslipidemia | 5 | 1.41 | Not true | Neutral | No consensus | |
| Type 2 diabetes mellitus | 5 | 1.24 | Not true | Neutral | No consensus | |
| Obesity | 7 | 1.12 | Appropriate | Neutral | No consensus | |
| Metabolic syndrome | 7 | 1.29 | Appropriate | Neutral | No consensus | |
| Liver disease | 7 | 1.35 | Appropriate | Neutral | No consensus | |
| Chronic kidney failure | 3 | 1.29 | Inappropriate | Neutral | No consensus | |
| Demyelinating disorders | 8 | 1.24 | Appropriate | Agree | Consensus in second round | |
| Inflammatory bowel disease | 5 | 1.29 | Not true | Neutral | No consensus | |
| Mood disorders | 2 | 1.12 | Inappropriate | Agree | Consensus in second round | |
| Multiple comorbidities | 7 | 1.29 | Appropriate | Neutral | No consensus | |
| Recurrent or chronic infections including HIV, HVB, and HVC infections and tuberculosis | 8 | 0.76 | Appropriate | Agree | Consensus in first round | |
| Active infections including HIV, HVB, and HVC infections and tuberculosis | 8 | 0.88 | Appropriate | Agree | Consensus in first round | |
| A recent history of cancer (< 5 y) | 9 | 0.47 | Appropriate | Agree | Consensus in first round | |
| Active cancer | 9 | 1.00 | Appropriate | Agree | Consensus in first round | |
| Other immunosuppressive states | 8 | 1.12 | Appropriate | Agree | Consensus in first round | |
| 23 | Screening before treatment initiation is not as necessary with apremilast as with other conventional systemic therapies. | 8 | 2.06 | Appropriate | Agree | Consensus in first round |
| 24 | Screening before treatment initiation is not as necessary with apremilast as with biologic therapies. | 9 | 1.35 | Appropriate | Agree | Consensus in first round |
| 25 | Screening is not necessary before treatment initiation with apremilast. | 6 | 1.94 | Not true | Disagree | No consensus |
| 26 | Screening before treatment initiation with apremilast is necessary in the following situations: | |||||
| Conventional blood tests are advisable before treatment initiation with apremilast. | 9 | 1.06 | Appropriate | Agree | Consensus in first round | |
| A complete blood test, including serology for viral infections (HIV, HVB, and HVC), is necessary before treatment initiation with apremilast. | 2 | 1.94 | Inappropriate | Neutral | No consensus | |
| A chest radiograph is necessary before treatment initiation with apremilast. | 2 | 1.29 | Inappropriate | Agree | Consensus in first round | |
| Latent tuberculosis testing is necessary before treatment initiation with apremilast. | 2 | 1.29 | Inappropriate | Agree | Consensus in first round | |
| A pregnancy test is advisable before treatment initiation with apremilast in women of childbearing age. | 9 | 1.65 | Appropriate | Agree | Consensus in first round | |
| It is not necessary to change a patient’s vaccine schedule after initiation of treatment with apremilast. | 9 | 1.18 | Appropriate | Agree | Consensus in first round |
Abbreviations: HIV, human immunodeficiency virus; HVB, hepatitis virus B; HVC, hepatitis virus C; MAD-M, mean absolute deviation from the median.
Prescreening and follow-up requirements were considered to be less stringent with apremilast than with conventional systemic or biologic therapies. The panel agreed, however, that blood tests and a pregnancy test in women of childbearing potential were advisable before treatment initiation. They did not, however, consider it necessary to perform a chest radiograph or to test for latent tuberculosis before starting treatment. It was also considered unnecessary to modify vaccine schedules once treatment had started (Table 5).
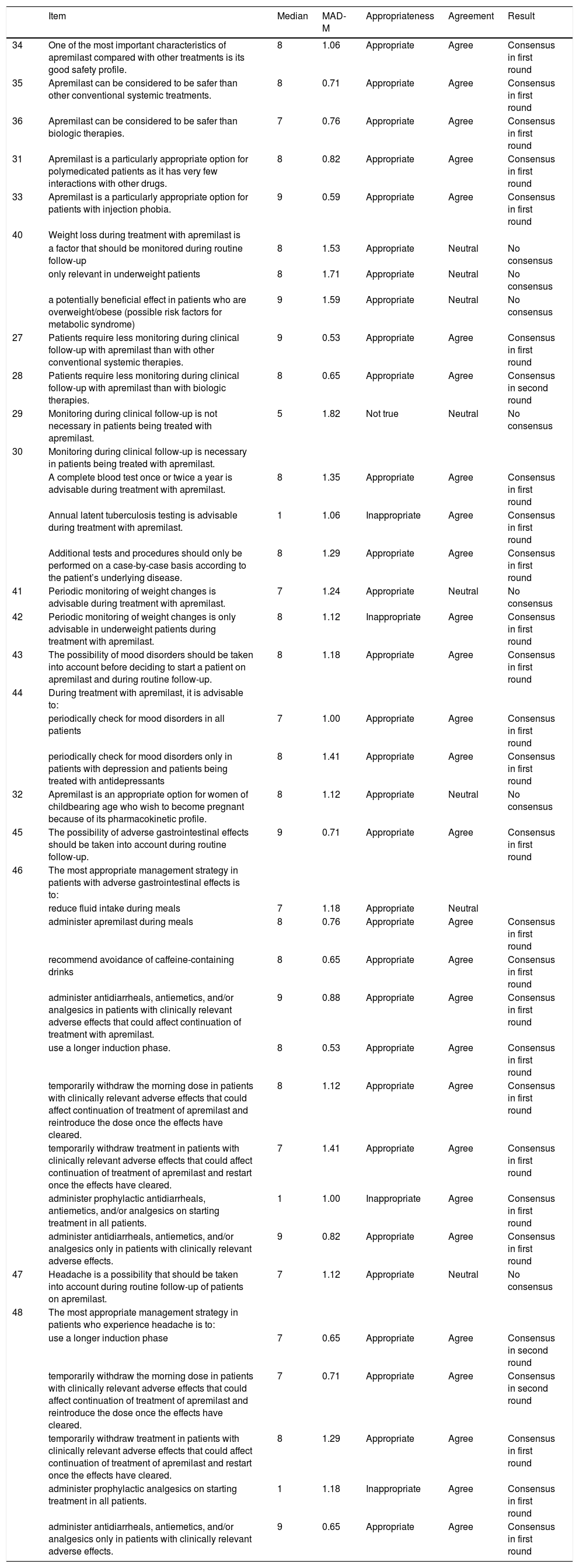
Safety IssuesPositive consensus was reached for 24 (66.7%) of the 36 items (17 in the first round and 7 in the second ) on safety issues associated with the use of apremilast. Negative consensus was achieved for 4 items (11.1%), all in the first round. Monitoring was considered less necessary during treatment with apremilast than with conventional systemic treatment (consensus reached in the first round) or biologic therapy (consensus reached in the second round). It was agreed that a complete blood test was advisable once or twice a year and that additional tests should be ordered in accordance with the patient’s underlying disease. The panel also agreed that apremilast was an appropriate option for polymedicated patients and patients with injection phobia.
Weight loss, mood changes, and gastrointestinal disorders were all considered to be important adverse effects that should be contemplated before starting treatment with apremilast and monitored periodically thereafter. The panel also recommended monitoring weight changes in underweight patients and stressed the need to consider the risk of mood changes before starting a patient on apremilast and to monitor for such changes during treatment. The recommendations for minimizing and managing adverse effects are summarized in Table 6.
Safety Issues Associated With the Use of Apremilast.
| Item | Median | MAD-M | Appropriateness | Agreement | Result | |
|---|---|---|---|---|---|---|
| 34 | One of the most important characteristics of apremilast compared with other treatments is its good safety profile. | 8 | 1.06 | Appropriate | Agree | Consensus in first round |
| 35 | Apremilast can be considered to be safer than other conventional systemic treatments. | 8 | 0.71 | Appropriate | Agree | Consensus in first round |
| 36 | Apremilast can be considered to be safer than biologic therapies. | 7 | 0.76 | Appropriate | Agree | Consensus in first round |
| 31 | Apremilast is a particularly appropriate option for polymedicated patients as it has very few interactions with other drugs. | 8 | 0.82 | Appropriate | Agree | Consensus in first round |
| 33 | Apremilast is a particularly appropriate option for patients with injection phobia. | 9 | 0.59 | Appropriate | Agree | Consensus in first round |
| 40 | Weight loss during treatment with apremilast is | |||||
| a factor that should be monitored during routine follow-up | 8 | 1.53 | Appropriate | Neutral | No consensus | |
| only relevant in underweight patients | 8 | 1.71 | Appropriate | Neutral | No consensus | |
| a potentially beneficial effect in patients who are overweight/obese (possible risk factors for metabolic syndrome) | 9 | 1.59 | Appropriate | Neutral | No consensus | |
| 27 | Patients require less monitoring during clinical follow-up with apremilast than with other conventional systemic therapies. | 9 | 0.53 | Appropriate | Agree | Consensus in first round |
| 28 | Patients require less monitoring during clinical follow-up with apremilast than with biologic therapies. | 8 | 0.65 | Appropriate | Agree | Consensus in second round |
| 29 | Monitoring during clinical follow-up is not necessary in patients being treated with apremilast. | 5 | 1.82 | Not true | Neutral | No consensus |
| 30 | Monitoring during clinical follow-up is necessary in patients being treated with apremilast. | |||||
| A complete blood test once or twice a year is advisable during treatment with apremilast. | 8 | 1.35 | Appropriate | Agree | Consensus in first round | |
| Annual latent tuberculosis testing is advisable during treatment with apremilast. | 1 | 1.06 | Inappropriate | Agree | Consensus in first round | |
| Additional tests and procedures should only be performed on a case-by-case basis according to the patient’s underlying disease. | 8 | 1.29 | Appropriate | Agree | Consensus in first round | |
| 41 | Periodic monitoring of weight changes is advisable during treatment with apremilast. | 7 | 1.24 | Appropriate | Neutral | No consensus |
| 42 | Periodic monitoring of weight changes is only advisable in underweight patients during treatment with apremilast. | 8 | 1.12 | Inappropriate | Agree | Consensus in first round |
| 43 | The possibility of mood disorders should be taken into account before deciding to start a patient on apremilast and during routine follow-up. | 8 | 1.18 | Appropriate | Agree | Consensus in first round |
| 44 | During treatment with apremilast, it is advisable to: | |||||
| periodically check for mood disorders in all patients | 7 | 1.00 | Appropriate | Agree | Consensus in first round | |
| periodically check for mood disorders only in patients with depression and patients being treated with antidepressants | 8 | 1.41 | Appropriate | Agree | Consensus in first round | |
| 32 | Apremilast is an appropriate option for women of childbearing age who wish to become pregnant because of its pharmacokinetic profile. | 8 | 1.12 | Appropriate | Neutral | No consensus |
| 45 | The possibility of adverse gastrointestinal effects should be taken into account during routine follow-up. | 9 | 0.71 | Appropriate | Agree | Consensus in first round |
| 46 | The most appropriate management strategy in patients with adverse gastrointestinal effects is to: | |||||
| reduce fluid intake during meals | 7 | 1.18 | Appropriate | Neutral | ||
| administer apremilast during meals | 8 | 0.76 | Appropriate | Agree | Consensus in first round | |
| recommend avoidance of caffeine-containing drinks | 8 | 0.65 | Appropriate | Agree | Consensus in first round | |
| administer antidiarrheals, antiemetics, and/or analgesics in patients with clinically relevant adverse effects that could affect continuation of treatment with apremilast. | 9 | 0.88 | Appropriate | Agree | Consensus in first round | |
| use a longer induction phase. | 8 | 0.53 | Appropriate | Agree | Consensus in first round | |
| temporarily withdraw the morning dose in patients with clinically relevant adverse effects that could affect continuation of treatment of apremilast and reintroduce the dose once the effects have cleared. | 8 | 1.12 | Appropriate | Agree | Consensus in first round | |
| temporarily withdraw treatment in patients with clinically relevant adverse effects that could affect continuation of treatment of apremilast and restart once the effects have cleared. | 7 | 1.41 | Appropriate | Agree | Consensus in first round | |
| administer prophylactic antidiarrheals, antiemetics, and/or analgesics on starting treatment in all patients. | 1 | 1.00 | Inappropriate | Agree | Consensus in first round | |
| administer antidiarrheals, antiemetics, and/or analgesics only in patients with clinically relevant adverse effects. | 9 | 0.82 | Appropriate | Agree | Consensus in first round | |
| 47 | Headache is a possibility that should be taken into account during routine follow-up of patients on apremilast. | 7 | 1.12 | Appropriate | Neutral | No consensus |
| 48 | The most appropriate management strategy in patients who experience headache is to: | |||||
| use a longer induction phase | 7 | 0.65 | Appropriate | Agree | Consensus in second round | |
| temporarily withdraw the morning dose in patients with clinically relevant adverse effects that could affect continuation of treatment of apremilast and reintroduce the dose once the effects have cleared. | 7 | 0.71 | Appropriate | Agree | Consensus in second round | |
| temporarily withdraw treatment in patients with clinically relevant adverse effects that could affect continuation of treatment of apremilast and restart once the effects have cleared. | 8 | 1.29 | Appropriate | Agree | Consensus in first round | |
| administer prophylactic analgesics on starting treatment in all patients. | 1 | 1.18 | Inappropriate | Agree | Consensus in first round | |
| administer antidiarrheals, antiemetics, and/or analgesics only in patients with clinically relevant adverse effects. | 9 | 0.65 | Appropriate | Agree | Consensus in first round |
Abbreviation: MAD-M, mean absolute deviation from the median.
The efficacy of apremilast in the treatment of moderate to severe plaque psoriasis has been evaluated in several phase II and III randomized clinical trials.
In the phase III ESTEEM 1 and 2 trials,2,3 one-third of patients in the apremilast arm achieved PASI-75 by week 16 compared with 6% in the placebo arm. Half of the patients maintained this response at week 50, and over 75% maintained a reduction in PASI of 70% or greater.
Outcomes from clinical trials, however, even when significant, do not necessarily reflect the use of conventional new-generation drugs such as apremilast in real-world clinical practice.
The expert panel agreed that apremilast was most likely to be successful in patients with stable, moderate psoriasis. There are, however, no clear or agreed-on definitions of moderate psoriasis. The 2011 American Academy of Dermatology guidelines of care for the management of psoriasis and psoriatic arthritis defined 3 categories of psoriasis according to percentage of body surface area (BSA) affected and considered that patients with a BSA involvement of between 5% and 10% had moderate psoriasis.8
In a survey of US dermatologists, Knuckles et al.9 found that most dermatologists considered a median BSA of 5% to 10% to indicate moderate psoriasis, although cutoff scores mentioned varied considerably. Llamas-Velasco et al.10 proposed that moderate psoriasis should be defined by either a PASI score of less than 7 and a DLQI score of at least 5 or a PASI score of between 7 and 15 regardless of DLQI; these latter scores overlap with scores indicating candidacy for biologic therapy. The prospective LAPIS-PSO study of 500 patients treated with apremilast in routine care found that patients were more likely to achieve PASI-75 or a physician global assessment score of 0 or 1 (PGA 0/1) when their baseline PASI was lower than that in the ESTEEM 1 and 2 trials.11,12 Papadavid et al., 13 in a series of 51 patients with a mean PASI score of 10.8 treated in everyday practice, reported a PASI-75 rate of 59.3% at week 16. Aragón-Miguel et al.14 and Lukoviek et al.15 also reported lower PASI scores (9.37 and 9.01, respectively) in real-life settings. Finally, in the real-world APRIL study,16 patients still on apremilast 6 months after initiating treatment had a lower mean baseline PASI score than those who discontinued treatment due to a lack of response (9.4 vs. 11.8).
Most of the members of the expert panel considered that apremilast is an appropriate option for patients in whom conventional treatment fails or is contraindicated, and that it should ideally precede biologic therapy. This treatment profile differs to that of the pivotal trials, where most patients had severe, extensive psoriasis that was often refractory to biologic therapy. The outcomes could therefore vary. In the ESTEEM-2 study, for example, PASI-75 rates were significantly lower in patients who had been previously treated with biologics (22.8%) than in patients who had never been treated with systemic therapy, whether biologic (31.9%) or conventional (33.3%).3
In addition, patients still on apremilast after 6 months had on average been treated with fewer systemic treatments (mean, 1.47) and biologic therapies (0.20) than those no longer taking apremilast (1.96 and 0.70, respectively). Twenty-seven percent of patients in this group had received biologic therapy. Apremilast was clearly positioned by the panel as a conventional therapy in psoriasis, which seems reasonable considering both its chemical nature and effectiveness and safety profile. However, because of its price, similar to that of drugs in the biologics segment, access to this drug has been strongly shaped by reimbursement policies.17 Despite the above considerations, it should be recalled that there are no clinical trial data for the specific use of apremilast in moderate psoriasis.
The expert panel considered that treatment effectiveness should be measured not just by objective indicators of complete or near-complete response (absolute PASI scores), but by a combination of indicators and other factors, in particular overall patient-reported satisfaction, tolerability, and safety. They also considered that a leeway of 24 weeks could be left before deciding whether or not to continue with treatment. This recommendation is in contrast to the treatment goals established in the clinical trials of apremilast, highlighting the challenges that lie ahead in terms of determining treatment goals and follow-up intervals for patients with psoriasis and also highlighting the role of patient empowerment in terms of the consideration of patient-reported outcome measures.18 Nonetheless, this recommendation also complicates the objective comparison of the drug’s effectiveness—and efficiency—relative to other treatments on the market.
Despite the limited evidence available, the expert panel considered that apremilast is a useful option for treating refractory disease in difficult-to-treat areas (palms/soles, nails, and scalp). This recommendation is supported by observations (limited by patient numbers) from phase II and III clinical trials.19–21 In the observational LAPIS-PSO study, 62% of patients treated with apremilast in routine care achieved nail PASI-50 after 4 months; in the same period, approximately 60% achieved scalp PGA 0/1 and almost 70% achieved palmoplantar PGA 0/111,22 (Appendix B Table S2. See supplementary material).
In the only randomized trial to specifically evaluate the efficacy of apremilast in palmoplantar psoriasis, Bissonnette et al.19 found no significant differences in PGA 0/1 between patients treated with apremilast and placebo after 16 weeks (4%, P = .1595). Nonetheless, 24% of patients treated with apremilast achieved this goal by week 32. In addition, patients in the apremilast arm performed better in terms of PASI-75 (22% vs. 8% for placebo, P = .0499) and DLQI (−4.3 [5.1] vs.−0.8 [4.5], P = .0004) scores. In the STYLE study, the first phase III multicenter, placebo-controlled, randomized clinical trial to evaluate the efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp, 43.4% of patients in the treatment arm versus 13.8% in the placebo arm achieved the primary endpoint, which was scalp PGA 0/1 with an improvement of 2 points or more from baseline to week 16.23
The panel considered that the concomitant presence of psoriatic arthritis constituted an additional reason to use apremilast, particularly in patients with moderate psoriasis, joint involvement, or dactylitis. The phase III PALACE-1-3 clinical trial assessed the efficacy of apremilast in the treatment of psoriatic arthritis.24–26 After 16 weeks of treatment, 40% of patients in the apremilast arm (30 mg twice daily) versus 20% of those in the placebo arm achieved a 20% or greater improvement in American College of Rheumatology response criteria.
Over 70% of patients treated with apremilast maintained this response in the long term (260 weeks).
Approximately 55% and 80% of patients who achieved complete resolution of enthesitis and dactylitis, respectively, remained stable on 30 mg of apremilast administered twice a day.26
Overall, the panel members considered safety to be one the most attractive attributes of apremilast. The most common adverse effects (affecting ≥ 5% of patients) are summarized in Appendix B Table S3 of the supplementary material.27,28 In the pooled safety analysis of the ESTEEM 1 and 2 trials, the incidence of adverse effects at 156 weeks was not significantly higher than that observed after 1 year for cardiovascular events (exposure-adjusted incidence rate of 0.5/100 patient-years), malignancies (1.2/100 patient-years), depression (1.8/100 patient-years), and suicide attempts (0.1/100 patient-years).28 Data from the observational LAPIS-PSO study, which analyzed 500 patients, suggested that the overall incidence of adverse events might be lower in routine care than in clinical trial settings.
The members of the expert panel considered that patients treated with apremilast need less rigorous prescreening and follow-up than patients treated with conventional systemic treatments or biologic therapy. A priori, this could be viewed as offering certain advantages over the standard protocol required for systemic therapies and could lead to greater prescribing of apremilast in centers where it takes time or considerable effort to gain access to conventional systemic treatments and biologic therapy. The summary of product characteristics (SPC) does not mention the need for special tests before or during treatment with apremilast.27
In addition to the contraindications described for apremilast in the SPC, the European S3-Guideline mentions acute, severe infections as an absolute contraindication for apremilast.29 It also includes acute and chronic infections, cancer, lymphoproliferative disorders, and depression as relative contraindications. There is no evidence on the use of apremilast in patients with cancer, as, with the exception of cervical intraepithelial neoplasia and squamous cell carcinoma, malignancy was an exclusion criterion in the clinical trials. Depression, aside from being a common comorbidity in patients with psoriasis,30,31 is listed as a potential adverse effect in the SPC for apremilast. Just over 1% of patients treated with apremilast in the phase III clinical trials and 0.5% of those treated with placebo experienced depression.27
Regarding follow-up recommendations, the panel considered that an annual blood test was sufficient and that other tests or procedures should be performed on an as-needed basis. This consideration is consistent with the recommendations of the European Academy of Dermatology and Venerology.29
The most relevant adverse events associated with apremilast according to the expert panel are gastrointestinal problems, mood changes, and weight loss. Supporting observations in the literature,32 it was agreed that awareness and prompt management of adverse effects can help minimize their impact and enable patients to continue with treatment. Nonetheless, treatment discontinuation due to tolerability problems is likely to be more common in real-world settings than in clinical trials, probably as patients treated in routine practice have access to other treatments. In a series of 77 patients treated with apremilast in a real-world setting, Lee et al.33 reported a treatment discontinuation rate of 23% over a mean period of 123 days, contrasting with the rate of 5.3% reported in the clinical trials. Treatment was stopped in most cases because of adverse effects.
In brief, the clinicians who participated in this study view apremilast as an attractive option for the individualized treatment of psoriasis and consider that objective indicators of effectiveness should be contemplated together with safety, convenience, and patient satisfaction. In particular, they appreciated the versatility of the drug as an option for patients who are not candidates for other treatments as well as its favorable safety profile and positive impact on symptoms, quality of life, and lesions in difficult-to-treat areas. Disadvantages that should be considered include its lower effectiveness compared with other new-generation drugs and certain tolerability aspects that could influence efficiency in this highly competitive setting.
This study highlights the importance of defining moderate psoriasis to enable comparative studies of apremilast and other drugs as well as the need for greater evidence from clinical trials and real-world settings to verify the recommendations of the experts.
Conflicts ofInterestJose Manuel Carrascosa has served as a consultant and speaker, participated in clinical trials, and attended meetings and conferences with financial support from AbbVie, Celgene, Janssen-Cilag, Leo-Pharma, Lilly, Novartis, Pfizer, Biogen, Sandoz, Milan, Sanofi-Aventis, and Almirall.
Mariano Ara has given talks and attended consultancy meetings sponsored by AbbVie, Pfizer, MSD, Janssen, Celgene, Almirall, Amgen, Novartis, Lilly, and Leo.
Pedro Herranz has worked as a consultant/speaker/researcher for AbbVie, Celgene, Lilly, Janssen-Cilag, MSD, Novartis, Pfizer, Regeneron, and Sanofi-Aventis
Isabel Belinchon has participated in clinical trials and received consultancy and speakers’ fees from AbbVie, Janssen, MSD, Pfizer-Wyeth, Leo, Celgene, Novartis, and Lilly.
Marta García-Bustínduy has served as a consultant and speaker, participated in clinical trials, and attended meetings and conferences with financial support from AbbVie, Celgene, Janssen-Cilag, Leo, Lilly, Novartis, MSD, Pfizer-Wyeth, and Sanofi-Aventis.
Celgene provided bibliographic support and collaborated with the organization of working meetings and the design and development of the Delphi study carried out by the independent scientific group. Nobody involved with Celgene participated in the generation of proposals or other content produced by this working group or in the writing of this manuscript.
AcknowledgementCelgene provided bibliographic support and collaborated with the organization of working meetings and the design and development of the Delphi study carried out by the independent scientific group.
Please cite this article as: Carrascosa JM, et al. Estudio Delphi para el uso de apremilast en la psoriasis. Actas Dermosifiliogr. 2020;111:115–134.