Nonmelanoma skin cancer (NMSC) is the most common malignancy in patients who have received a solid organ transplant. Multiple factors are involved in the onset of posttransplant NMSC.
ObjectivesTo analyze the relationship between new immunosuppressive drugs and the onset of NMSC in renal transplant recipients.
MethodThis was a combined retrospective and prospective observational study in which we studied 289 patients who received a kidney transplant between January 1996 and December 2010 at Hospital Universitario Doctor Peset in Valencia, Spain.
ResultsSeventy-three patients (25.2%) developed 162 NMSCs over a median follow-up of 72 months. There were no statistically significant differences in the onset of NMSC on comparing different induction therapy strategies involving monoclonal and polyclonal antibodies. NMSCs occurred less frequently in patients treated with mammalian target of rapamycin (mTOR) inhibitors than in those treated with other immunosuppressive regimens, although the differences were not statistically significant. Three of 5 patients with recurrent NMSC who were switched from calcineurin inhibitors to mTOR inhibitors developed additional NMSCs despite the change.
ConclusionsInduction therapy with monoclonal and polyclonal antibodies in renal transplant recipients is not associated with an increased risk of NMSC. While mTOR inhibitors are associated with a lower risk of posttransplant NMSC, it remains to be determined whether a switch to these drugs is useful in the management of patients who develop multiple NMSCs.
El cáncer de piel no melanoma (CCNM) es la neoplasia maligna que se presenta con más frecuencia después de un trasplante de órgano sólido. La etiología del CCNM tras el trasplante es multifactorial.
ObjetivosAnalizar la relación entre los nuevos agentes inmunosupresores y la aparición de CCNM en pacientes trasplantados renales.
MétodoEstudio observacional. Se examinaron una combinación de datos retrospectivos y prospectivos. Incluimos en el estudio 289 pacientes que habían recibido trasplante renal desde enero de 1996 hasta diciembre de 2010 en el Hospital Universitario Doctor Peset de Valencia.
ResultadosTras una mediana de seguimiento de 72 meses 73 pacientes (25,2%) desarrollaron 162 CCNM. No hubo diferencias estadísticamente significativas en la incidencia de CCNM al comparar las distintas estrategias de inducción con anticuerpos mono o policlonales. La incidencia de tumores en pacientes con inhibidores mTOR fue menor que con el resto de tratamientos, aunque sin mostrar diferencias estadísticamente significativas. De 5 pacientes con CCNM recurrente que pasaron a tratarse con inhibidores mTOR (tras ser tratados previamente con inhibidores de la calcineurina), 3 continuaron presentando CCNM a pesar del cambio de tratamiento.
ConclusionesLa utilización de anticuerpos mono o policlonales en la terapia de inducción en pacientes trasplantados renales no se asocia a un mayor riesgo de CCNM. Si bien los inhibidores mTOR muestran menor riesgo de aparición de CCNM postrasplante queda por determinar si el cambio de tratamiento a inhibidores mTOR es un buena opción en el manejo de pacientes con múltiples CCNM.
Nonmelanoma skin cancer (NMSC) is the most common malignancy in patients who have received a solid organ transplant.1–3 Multiple factors are involved in the onset of posttransplant NMSC. In addition to ultraviolet UV radiation, other factors such as infection with human papillomavirus, genetics, and immunosuppressive therapy play an important role in the onset of these tumors.4–6
Immunosuppressive therapy is widely recognized as a risk factor in the etiology and pathogenesis of NMSC. Skin cancer arises as a result of decreased immunologic activity as well as direct oncogenic effects linked to certain immunosuppressive agents, although it is difficult to determine which mechanism is predominant.
Several studies have suggested that calcineurin inhibitors (tacrolimus and ciclosporin) have oncogenic properties, primarily linked to the production of cytokines that promote tumor growth and angiogenesis.7–9 Treatment with azathioprine photosensitizes human skin to UV radiation10 by promoting mutagenic DNA changes through 6-thioguanine, a metabolite of the drug. However, studies in humans11,12 have demonstrated that the mammalian target of rapamycin (mTOR) inhibitors sirolimus and everolimus exert antineoplastic effects through multiple mechanisms: blocking of angiogenesis, inhibition of cell replication, inhibition of interleukin 10, and induction of apoptosis. mTOR inhibitors have thus been shown to have a preventive action against skin carcinogenesis as well as antitumor effects after the onset of malignant cutaneous tumors.
Immunosuppressive therapy in renal transplant recipients must be understood as a dynamic process that is constantly adapted to the characteristics of the patient's disease progression. In this context, immunosuppressive therapy is considered to be an induction therapy—i.e., introduced immediately following a transplant—and also a long-term maintenance therapy. Because of the increased risk of acute rejection during induction therapy, immunosuppressive therapy must be more powerful and intense during this period. The combination of immunosuppressive drugs used is essential. Induction therapy can involve monoclonal antibodies (muromonab-CD3 [OKT3] and basiliximab [Simulect]) or polyclonal antibodies (equine antithymocyte globulin [ATGAM] or rabbit antithymocyte globulin [Thymoglobulin]). These drugs are administered intravenously, generally during the first 10 to 15 days after the transplant, together with other immunosuppressive drugs (corticosteroids, calcineurin inhibitors, and mycophenolate mofetil). mTOR inhibitors are not usually administered during the immediate postoperative period (in order to avoid adverse effects related to the drugs’ antiproliferative activity and problems related to surgical wound healing) and are instead introduced a few weeks after the transplant. The use of induction therapies with polyclonal or monoclonal antibodies was previously restricted in patients with high immunologic risk (hypersensitized patients and second transplant recipients). However, the trend since 2009 has been to use induction therapies in all cases.13 Basiliximab, a monoclonal antibody that binds to the α subunit (CD25) of the interleukin 2 receptors, is the recommended drug in patients with low immunologic risk. Lymphocyte-depleting agents (muromonab-CD3 and polyclonal antibodies) are reserved for patients with a high immunologic risk. After the initial induction stage, the risk of acute rejection gradually decreases and the patient begins to face new risks such as chronic rejection, cardiovascular morbidity and mortality, and the development of cancer. During the maintenance phase, immunosuppressive drugs should be gradually withdrawn and/or their dosages should be decreased with the goal of maintaining low doses of 1 or 2 immunosuppressive drugs (generally tacrolimus or an mTOR inhibitor plus mycophenolate mofetil) that achieve a synergistic immunosuppressive effect and a better safety profile.
The main objective of this study was to analyze the relationship between new immunosuppressive drugs (monoclonal and polyclonal antibodies in induction therapy and mTOR inhibitors in maintenance therapy) and the onset of NMSC in renal transplant recipients. The secondary objective was to investigate the antitumor effect of mTOR inhibitors following a switch from calcineurin inhibitors to these drugs in renal transplant recipients with multiple NMSCs.
Patients and MethodsIn this ambispective observational study, we used retrospective data (patient record review and structured questionnaire) as well as prospective data collected during the recruitment period and in periodic reviews throughout the study.
We collected data on all patients who underwent a renal transplant at the Hospital Universitario Doctor Peset in Valencia, Spain, from January 1996 to December 2010. A total of 622 renal transplants were performed at the hospital during this period. The renal transplant department invited all patients to enroll voluntarily from January 2010 until the end of the study in January 2012. The recruited patients completed a structured questionnaire. They also underwent a complete skin examination by the same dermatologist and attended follow-up appointments every 6 months until the end of the study.
The hospital ethics committee approved the study, and written informed consent was obtained from each patient.
In our review of patient records, we collected the following data: year of transplant, age at the time of transplant, skin phototype (Fitzpatrick scale, I-VI), pretransplant occupational sun exposure (considered to be high if the patient spent most of the work day outdoors), NMSCs diagnosed to date, and posttransplant immunosuppressive therapy. During follow-up, all suspected skin cancer lesions were excised and/or biopsied and analyzed histologically. Only histologically diagnosed NMSCs were included in the study.
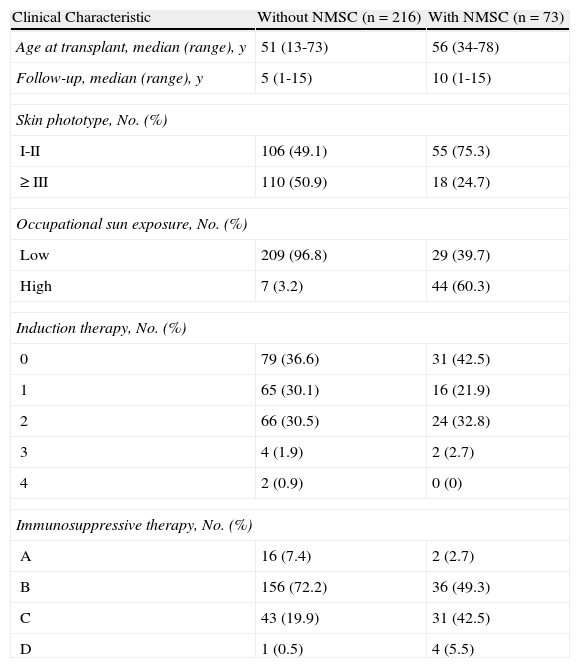
The patients were divided into 5 groups as a function of the monoclonal or polyclonal antibodies they received during induction therapy: group 0, no induction therapy with any of the studied drugs; group 1, induction therapy with basiliximab (Simulect) (Novartis, Barcelona, Spain); group 2, induction therapy with rabbit antithymocyte globulin (Thymoglobulin) (Genzyme Europe BV, Naarden, The Netherlands); group 3, induction therapy with equine antithymocyte globulin (ATGAM) (Pfizer, Madrid, Spain); and group 4, induction therapy with muromonab-CD3 (OKT3) (Janssen, Madrid, Spain) (Table 1).
Clinical Characteristics of Renal Transplant Recipients.
| Clinical Characteristic | Without NMSC (n = 216) | With NMSC (n = 73) |
| Age at transplant, median (range), y | 51 (13-73) | 56 (34-78) |
| Follow-up, median (range), y | 5 (1-15) | 10 (1-15) |
| Skin phototype, No. (%) | ||
| I-II | 106 (49.1) | 55 (75.3) |
| ≥III | 110 (50.9) | 18 (24.7) |
| Occupational sun exposure, No. (%) | ||
| Low | 209 (96.8) | 29 (39.7) |
| High | 7 (3.2) | 44 (60.3) |
| Induction therapy, No. (%) | ||
| 0 | 79 (36.6) | 31 (42.5) |
| 1 | 65 (30.1) | 16 (21.9) |
| 2 | 66 (30.5) | 24 (32.8) |
| 3 | 4 (1.9) | 2 (2.7) |
| 4 | 2 (0.9) | 0 (0) |
| Immunosuppressive therapy, No. (%) | ||
| A | 16 (7.4) | 2 (2.7) |
| B | 156 (72.2) | 36 (49.3) |
| C | 43 (19.9) | 31 (42.5) |
| D | 1 (0.5) | 4 (5.5) |
Abbreviations: NMSC, nonmelanoma skin cancer; 0, no induction therapy; 1, basiliximab; 2, rabbit antithymocyte globulin; 3, equine antithymocyte globulin; 4, muromonab-CD3; A, mTOR inhibitors; B, tacrolimus; C, ciclosporin; D, azathioprine.
The patients were then divided into 4 groups as a function of the immunosuppressive maintenance therapy they received after the transplant: group A, mTOR inhibitor (sirolimus and/or everolimus)+mycophenolate mofetil; group B, tacrolimus+mycophenolate mofetil; group C, ciclosporin+mycophenolate mofetil; and group D, older regimens with azathioprine (Table 1). It is difficult to group transplant recipients by therapy because regimens tend to vary over time. We based our classifications on the therapy that each patient followed for the longest time.
Statistical AnalysisWe statistically analyzed the relationship between skin cancer development and induction therapy with monoclonal or polyclonal antibodies and immunosuppressive maintenance therapy. We also analyzed the relationship between NMSC and age at the time of transplant, skin phototype, and pretransplant occupational sun exposure as potential confounding factors for NMSC.
Continuous variables are expressed as medians and ranges. Nominal variables are expressed as absolute values and percentages.
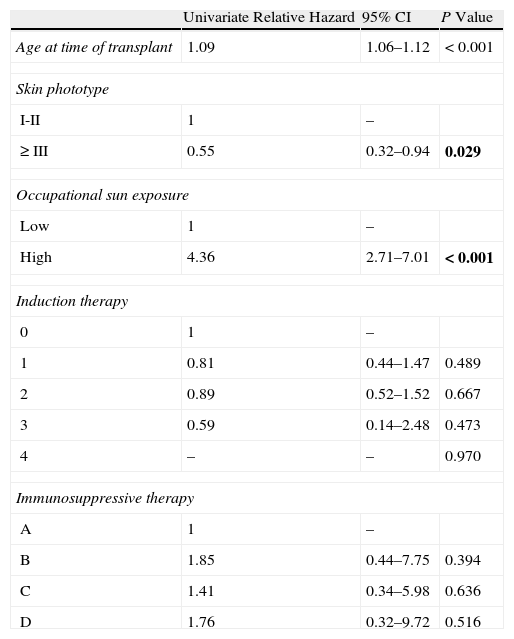
The statistical relationship between NMSC development and the aforementioned variables was assessed by means of univariate and multivariate analysis using the Cox proportional hazards model. The data were analyzed using the SPSS statistical package (version 19).
ResultsThe median follow-up time for the 289 patients included in the study was 72 months (range, 12–180 months).
The median age at the time of transplant was 52.2 years. The clinical characteristics of the patients are shown in Table 1.
Of the 289 patients, 73 (25.2%) developed 162 tumors, of which 41 were squamous cell carcinomas (SCCs), 91 were basal cell carcinomas (BCCs), 25 were Bowen disease, and 4 were keratoacanthomas. The ratio of BCCs to SCCs was 2.2:1.
Of the 179 (61.9%) patients who received induction therapy, 81 received basiliximab, 90 received rabbit antithymocyte globulin, 6 received equine antithymocyte globulin, and 2 received muromonab-CD3. We found no differences in the incidence of NMSC between the various induction therapy drug groups or the group that received no induction therapy (Table 2).
Univariate Analysis of Risk Factors for Nonmelanoma Skin Cancer.a
| Univariate Relative Hazard | 95% CI | P Value | |
| Age at time of transplant | 1.09 | 1.06–1.12 | <0.001 |
| Skin phototype | |||
| I-II | 1 | – | |
| ≥III | 0.55 | 0.32–0.94 | 0.029 |
| Occupational sun exposure | |||
| Low | 1 | – | |
| High | 4.36 | 2.71–7.01 | <0.001 |
| Induction therapy | |||
| 0 | 1 | – | |
| 1 | 0.81 | 0.44–1.47 | 0.489 |
| 2 | 0.89 | 0.52–1.52 | 0.667 |
| 3 | 0.59 | 0.14–2.48 | 0.473 |
| 4 | – | – | 0.970 |
| Immunosuppressive therapy | |||
| A | 1 | – | |
| B | 1.85 | 0.44–7.75 | 0.394 |
| C | 1.41 | 0.34–5.98 | 0.636 |
| D | 1.76 | 0.32–9.72 | 0.516 |
Abbreviations: CI, confidence interval; 0, no induction; 1, basiliximab; 2, rabbit antithymocyte globulin; 3, equine antithymocyte globulin; 4, muromonab-CD3; A, mTOR inhibitors; B, tacrolimus; C, ciclosporin; D, azathioprine.
Analysis of the various immunosuppressive maintenance therapies revealed that group A (mTOR inhibitors+mycophenolate mofetil) had a lower risk of NMSC in the univariate analysis, although this finding was not statistically significant (Table 2).
During follow-up, 13 patients receiving treatment with calcineurin inhibitors switched to mTOR inhibitors (5 switched because of the onset of multiple NMSCs and 8 because of the toxic effects of tacrolimus). Of the 5 patients with multiple NMSCs who switched to mTOR inhibitors, 3 continued to develop NMSCs after 24 months of follow-up despite the switch. None of the 8 patients who switched to mTOR inhibitors because of the toxic effects of tacrolimus developed NMSCs either before or after the switch.
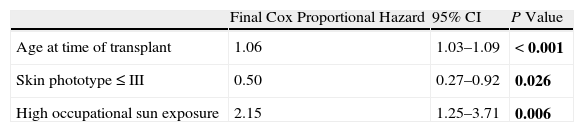
In the multivariate analysis, only age at the time of transplant, skin phototype, and occupational sun exposure had a statistically significant association with skin cancer risk (Table 3).
Multivariate Analysis of Risk Factors for Nonmelanoma Skin Cancer.a
| Final Cox Proportional Hazard | 95% CI | P Value | |
| Age at time of transplant | 1.06 | 1.03–1.09 | <0.001 |
| Skin phototype ≤ III | 0.50 | 0.27–0.92 | 0.026 |
| High occupational sun exposure | 2.15 | 1.25–3.71 | 0.006 |
Abbreviation: CI, confidence interval.
The use of monoclonal or polyclonal antibodies—especially muromonab-CD3—in induction therapy in transplant recipients has been associated with neoplasms, specifically lymphoproliferative disorders and skin tumors.14–16 Caillard et al.15 found that treatment with equine or rabbit antithymocyte globulin and muromonab-CD3 was associated with a higher risk of lymphoproliferative disorders 3 years after transplant. Opelz et al.14 observed that induction therapy with muromonab-CD3 or antithymocyte globulin increased the risk of lymphoma during the first year after transplant, but that induction therapy with basiliximab did not. Bustamini et al.16 found that induction therapy with muromonab-CD3, antithymocyte globulin, and basiliximab was associated with a significantly higher relative risk of lymphoproliferative disorders.
The role of these drugs in the onset of skin cancer has been the subject of fewer studies and is therefore less well defined. Lampros et al.17 found that treatment with muromonab-CD3 was associated with an increased risk of skin cancer. In a study by Molina et al.,18 therapy with muromonab-CD3 was found to be associated with an increased risk of BCC and SCC, whereas therapy with antithymocyte globulins was found to be associated with an increased risk of BCC but not SCC. Interestingly, Wimmer et al.19 found that the use of basiliximab significantly reduced the risk of tumors in transplant recipients.
In our study, a comparison of various induction strategies involving these drugs did not reveal any statistically significant differences in the incidence of NMSC. However, because only 2 patients received induction therapy with muromonab-CD3, we are unable to draw significant conclusions with regard to this group. Similar results were obtained by Geusau et al.20 On comparing their own findings with data from other transplant centers where induction therapy is not usually used or where it is only included in treatment modalities used during periods of acute rejection, these authors found that induction therapy was not associated with a higher incidence of NMSC.
With regard to immunosuppressive maintenance therapy, the risk of skin cancer was higher in group A (mTOR inhibitors plus mycophenolate mofetil) than in the groups that received other drugs; however, the difference was not statistically significant, probably because of the small sample size of the group. These results are consistent with earlier studies that suggested that mTOR inhibitors could have a preventive effect on carcinogenesis.11,21,22 According to a recent report21 on 5 multicenter studies, maintenance immunosuppressive therapy with sirolimus was associated with a decrease in the incidence of malignancy 2 years after transplant. Kauffman et al.11 found a lower incidence of skin cancer after 2.6 years in patients who received mTOR inhibitors compared with patients who received calcineurin inhibitors. In a recent meta-analysis,23 the same antitumor effects were also observed in patients treated with sirolimus. In our study, the only clear trend toward a lower incidence of NMSC was found in patients treated with mTOR inhibitors. Further cohort studies with sufficient statistical power are needed in order to confirm this trend.
The antitumor effects of mTOR inhibitors have also been observed after the onset of malignant skin tumors, although these effects have most commonly been studied in patients with Kaposi sarcoma. Various studies24–26 have described the regression of Kaposi sarcoma following a switch to sirolimus in patients previously treated with other groups of immunosuppressants. As for malignant skin tumors, de Fijter et al.27 examined the use of mTOR inhibitors in 53 renal transplant recipients who developed NMSCs after the transplant. Of the 37 patients in whom regression of NMSC was observed, 15 developed new lesions following conversion to mTOR inhibitors. Of the 15 patients who developed new lesions, 2 were receiving low doses of calcineurin inhibitors as part of their immunosuppressive therapy regimen. In a study by Fernández et al.,28 9 renal transplant recipients were switched to everolimus because of the presence of malignancies or neurologic tacrolimus toxicity. In 6 patients with recurrent skin cancer who were switched from tacrolimus or ciclosporin to everolimus, no further skin tumors appeared during 6.5 months of follow-up. In a study by Euvrard et al.,29 an antitumor effect was observed after conversion to mTOR inhibitors in patients with a history of SCC who had previously been treated with calcineurin inhibitors. Salgo et al.30 reported that conversion to sirolimus induced regression of preexisting premalignant lesions and reduced the incidence of new NMSCs.
In our study, in contrast, NMSCs persisted after 24 months of follow-up in 3 of the 5 patients with multiple NMSCs who switched from calcineurin inhibitors to mTOR inhibitors. These findings could be explained by the older age and immunosuppressive load of the patients.
The limitations of this study include the biases introduced by the largely retrospective study design and the lack of uniformity in sample size among the various treatment groups. Similarly, as mentioned above, it was difficult to group transplant recipients by therapy because the treatment regimens tended to be changed over time as they lost efficacy or as adverse effects appeared.
In conclusion, we would like to highlight the high incidence of NMSC in our patients and the important role played by immunosuppressive therapy in the etiology and pathogenesis of these tumors. In order to reduce the tumor burden in these patients, their management requires a multidisciplinary approach that includes revision of immunosuppression. Monoclonal and polyclonal antibodies in induction therapy were not associated with a higher risk of posttransplant NMSC. Although a considerable amount of data suggests that the use of mTOR inhibitors is associated with reduced risk of posttransplant malignancy, it remains to be determined whether a switch to these drugs can modify the biological behavior of tumors in patients with recurrent NMSCs. These patients should therefore undergo periodic skin examinations and various clinical and environmental factors should be assessed as part of follow-up in transplant recipients.
Ethical DisclosuresProtection of persons and animalsThe authors declare that no experiments were performed on humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their hospitals concerning the publication of patient data and that all the patients included in this study were appropriately informed and gave their written informed consent.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Bernat-García J, Morales Suárez-Varela M, Vilata-Corell J, Marquina-Vila A, Pallardo L, Crespo J. Papel de los nuevos agentes inmunosupresores en el cáncer cutáneo no melanoma en pacientes trasplantados renales. Actas Dermosifiliogr. 2014;105:940–946.