Primary cutaneous lymphomas are uncommon. This article describes the Primary Cutaneous Lymphoma Registry of the Spanish Academy of Dermatology and Venereology (AEDV) and reports on the results from the first year.
Patients and methodsDisease registry for patients with primary cutaneous lymphoma. The participating hospitals prospectively recorded data on diagnosis, treatment, tests, and disease stage for all patients with primary cutaneous lymphoma. A descriptive analysis was performed.
ResultsIn December 2017, the registry contained data on 639 patients (60% male) from 16 university hospitals. The most common diagnoses, in order of frequency, were mycosis fungoides/Sézary syndrome (MF/SS) (348 cases, 55%), primary cutaneous B-cell lymphoma (CBCL) (184 cases, 29%), primary cutaneous CD30+ T-cell lymphoproliferative disorder (CD30+ CLPD) (70 cases, 11%), and other types of T-cell lymphoma (37 cases, 5%). In total, 105 (16.5%) of the cases recorded were incident cases. The most common diagnosis in the MF/SS group was classic MF (77.3%). Half of the patients with MF had stage IA disease when diagnosed, and the majority were either in partial remission (32.5%) or had stable disease (33.1%). The most widely used treatments were topical corticosteroids (90.8%) and phototherapy. The most common form of primary CBCL was marginal zone lymphoma (50%). Almost all of the patients had cutaneous involvement only and nearly half had stage T1a disease. Most (76.1%) were in complete remission. The main treatments were surgery (55.4%) and radiotherapy (41.9%). The most common diagnosis in patients with CD30+ CLPD was lymphomatoid papulosis (68.8%). Most of the patients (31.4%) had stage T3b disease and half were in complete remission. The most common treatments were topical corticosteroids (68.8%) and systemic chemotherapy (32.9%).
ConclusionThe characteristics of patients with primary cutaneous lymphoma in Spain do not differ from those described in other series in the literature. The registry will facilitate clinical research by the AEDV's lymphoma group.
Los linfomas primarios cutáneos son enfermedades poco frecuentes. Este artículo describe el Registro de linfomas cutáneos primarios de la AEDV y sus primeros resultados.
Pacientes y métodosRegistro de enfermedad de pacientes con linfomas cutáneos primarios. Los centros participantes recogieron datos prospectivamente de todos los pacientes, incluyendo datos del diagnóstico, de los tratamientos, de las pruebas realizadas y del estado actual del paciente. Se realizó un análisis descriptivo.
ResultadosEn diciembre del 2017 el registro tenía datos de 639 pacientes pertenecientes a 16 hospitales universitarios. Un 60% eran hombres y los diagnósticos, por orden de frecuencia, fueron: micosis fungoide/síndrome de Sézary (MF/SS) (348 casos; 55%), linfoma cutáneo primario de células B (LCCB) (184; 29%), trastorno linfoproliferativo de células T CD30+ (LTCD30+) (70; 11%) y otro tipo de linfoma T (OLT) (37; 5%). El número de casos incidentes recogidos durante el primer año fue de 105 (16,5%). En los pacientes con MF/SS, el diagnóstico más frecuente fue MF clásica (77,3%). La mitad de estos casos se diagnosticaron en estadio IA. La mayoría de los pacientes estaban en remisión parcial (32,5%) o enfermedad estable (33,1%). Los tratamientos más usados fueron los corticoides tópicos (90,8%) seguidos de fototerapia. En los pacientes con LCCB el diagnóstico más frecuente fue el linfoma de la zona marginal (50%). Casi todos los pacientes tuvieron afectación exclusivamente cutánea y casi la mitad fue T1a. La mayoría (76,1%) estaba en remisión completa. Los tratamientos más utilizados fueron la cirugía (55,4%) y la radioterapia (41,9%). En los pacientes con LTCD30+, el diagnóstico más frecuente fue la papulosis linfomatoide (68,6%). La mayoría fueron clasificados T3b (31,4%). La mitad de los casos estaban en remisión completa. Los tratamientos más frecuentes fueron los esteroides tópicos (68,6%), seguidos de la quimioterapia sistémica (32,9%).
ConclusiónLas características del paciente con linfoma cutáneo primario en España no difieren de otras series descritas en la literatura. El registro facilitará al grupo de linfomas de la AEDV realizar investigación clínica.
Primary cutaneous lymphomas are a heterogeneous group of extranodal lymphomas that show particular tropism for the skin, although they can spread to the lymph nodes and other organs during the course of disease. Depending on their origin, they are classified as T-cell, B-cell, or natural killer–cell lymphomas.1
Primary cutaneous lymphomas are rare, potentially serious tumors that require multidisciplinary management by an experienced team. Registries of diseases with a low prevalence, such as these, are useful.
The Spanish Academy of Dermatology and Venereology (AEDV) launched Spain's first Primary Cutaneous Lymphoma Registry in 2016. The initiative was promoted by the AEDV's Healthy Skin Foundation, which is formed by different hospitals that have dedicated units for diagnosing and managing these tumors. The aim is to build an active registry of patients with primary cutaneous lymphoma in Spain to facilitate epidemiological and disease monitoring studies and maintain an up-to-date record of patients who could participate in prospective studies.2
In this article, we present the registry and describe the basic clinical and management characteristics of the cases included in this first year of operation.
Patients and MethodsThe AEDV's Primary Cutaneous Lymphoma Registry is a prospective multicenter registry, although its initial design was ambispective, with the inclusion of both prevalent and incident cases. Patients qualified as prevalent cases if they had not been lost to follow-up and were scheduled for a check-up visit. All the participating hospitals have a specific cutaneous lymphoma unit and, to be included, patients must fulfil the diagnostic criteria proposed by the World Health Organization (WHO).1 The participating hospitals added patients consecutively diagnosed with cutaneous lymphoma to the registry. The only exclusion criterion was the patient's refusal to participate.
The information was entered into the database using the electronic software system OpenClinica (version 3.1). The study was classified as a non-postauthorization study by the Spanish Agency of Medicines and Medical Devices (AEMPS) and was approved by the ethics committee at Hospital 12 de Octubre (16/175).
The registry has a quality control system that consists of prior online training for all participating researchers and continuous checking of data through an online monitoring system.
The data in the registry are collected at baseline (inclusion) and follow-up visits. Data from the inclusion visit include demographics and diagnostic information, such as date of diagnosis, type of lymphoma according to the WHO classification system, and stage according to the TNM classification system proposed by the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC).3,4 A record is also made of diagnostic tests and treatments during follow-up.
The following data were collected during follow-up visits: date of last visit; disease status (complete remission [100% clearance since last visit], partial remission [50%-99% clearance since last visit], stable disease [< 25% to < 50% clearance since last visit], or progressive disease (≥ 25% progression since last visit); death or relapse; and current nodal, visceral, or blood involvement.
The patients were classified into 4 major groups: mycosis fungoides/Sézary syndrome (MF/SS), primary cutaneous CD30+ T-cell lymphoproliferative disorder (CD30+ CLPD), other types of T-cell lymphoma (OTCL), and primary cutaneous B-cell lymphoma (CBCL). We performed a descriptive analysis using absolute and relative frequencies for qualitative variables, means (SD) for normally distributed continuous variables, and medians (interquartile range [IQR]) for nonnormally distributed continuous variables.
ResultsAt the time of the analysis, December 2017, the registry contained data for 639 patients from 16 university hospitals (383 men and 256 women). Overall, 348 patients (55%) had MF/SS, 70 (11%) had CD30+ CLPD, 37 (6%) had OTCL, and 184 (29%) had CBCL. A total of 105 incident cases (16.5%) were recorded during the first year of the registry.
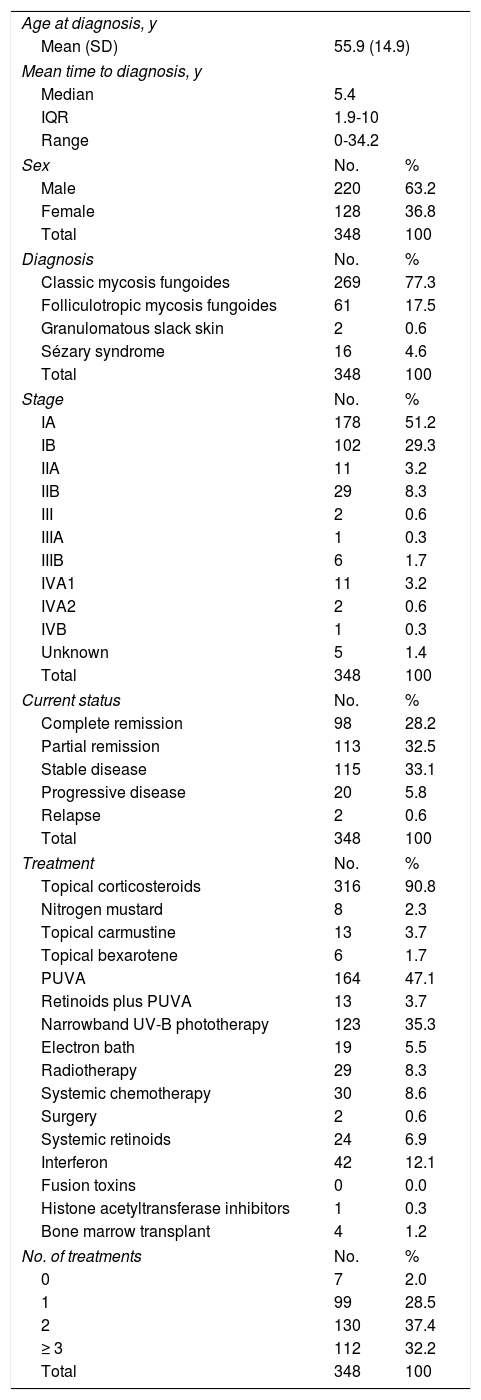
MF/SS GroupThe mean (SD) age at diagnosis in the MF/SS group was 55.9 (14.9) years, with a median time to diagnosis of 5.4 (IQR, 5.4-10) years and a male to female ratio of 1:1.7. The most common diagnosis was classic MF (77.3% of all cases); 4.6% of patients had SS. Over half of the patients with MF had stage IA disease at diagnosis and just 14.9% had advanced disease (> IIB). At the time of the last visit, the majority of patients had stable disease (37%) or were in partial remission (32.5%). The main treatment was topical corticosteroids (90.8%) followed by phototherapy. Most patients received more than 1 treatment (2 treatments, 37.4% and ≥3 treatments, 32.2%). The characteristics of the patients in the MF/SS group are summarized in Table 1.
Clinical Characteristics and Treatment of Patients With Mycosis Fungoides/Sézary Syndrome.
| Age at diagnosis, y | ||
| Mean (SD) | 55.9 (14.9) | |
| Mean time to diagnosis, y | ||
| Median | 5.4 | |
| IQR | 1.9-10 | |
| Range | 0-34.2 | |
| Sex | No. | % |
| Male | 220 | 63.2 |
| Female | 128 | 36.8 |
| Total | 348 | 100 |
| Diagnosis | No. | % |
| Classic mycosis fungoides | 269 | 77.3 |
| Folliculotropic mycosis fungoides | 61 | 17.5 |
| Granulomatous slack skin | 2 | 0.6 |
| Sézary syndrome | 16 | 4.6 |
| Total | 348 | 100 |
| Stage | No. | % |
| IA | 178 | 51.2 |
| IB | 102 | 29.3 |
| IIA | 11 | 3.2 |
| IIB | 29 | 8.3 |
| III | 2 | 0.6 |
| IIIA | 1 | 0.3 |
| IIIB | 6 | 1.7 |
| IVA1 | 11 | 3.2 |
| IVA2 | 2 | 0.6 |
| IVB | 1 | 0.3 |
| Unknown | 5 | 1.4 |
| Total | 348 | 100 |
| Current status | No. | % |
| Complete remission | 98 | 28.2 |
| Partial remission | 113 | 32.5 |
| Stable disease | 115 | 33.1 |
| Progressive disease | 20 | 5.8 |
| Relapse | 2 | 0.6 |
| Total | 348 | 100 |
| Treatment | No. | % |
| Topical corticosteroids | 316 | 90.8 |
| Nitrogen mustard | 8 | 2.3 |
| Topical carmustine | 13 | 3.7 |
| Topical bexarotene | 6 | 1.7 |
| PUVA | 164 | 47.1 |
| Retinoids plus PUVA | 13 | 3.7 |
| Narrowband UV-B phototherapy | 123 | 35.3 |
| Electron bath | 19 | 5.5 |
| Radiotherapy | 29 | 8.3 |
| Systemic chemotherapy | 30 | 8.6 |
| Surgery | 2 | 0.6 |
| Systemic retinoids | 24 | 6.9 |
| Interferon | 42 | 12.1 |
| Fusion toxins | 0 | 0.0 |
| Histone acetyltransferase inhibitors | 1 | 0.3 |
| Bone marrow transplant | 4 | 1.2 |
| No. of treatments | No. | % |
| 0 | 7 | 2.0 |
| 1 | 99 | 28.5 |
| 2 | 130 | 37.4 |
| ≥ 3 | 112 | 32.2 |
| Total | 348 | 100 |
Abbreviations: IQR, interquartile range; PUVA, psoralen plus UV-A.
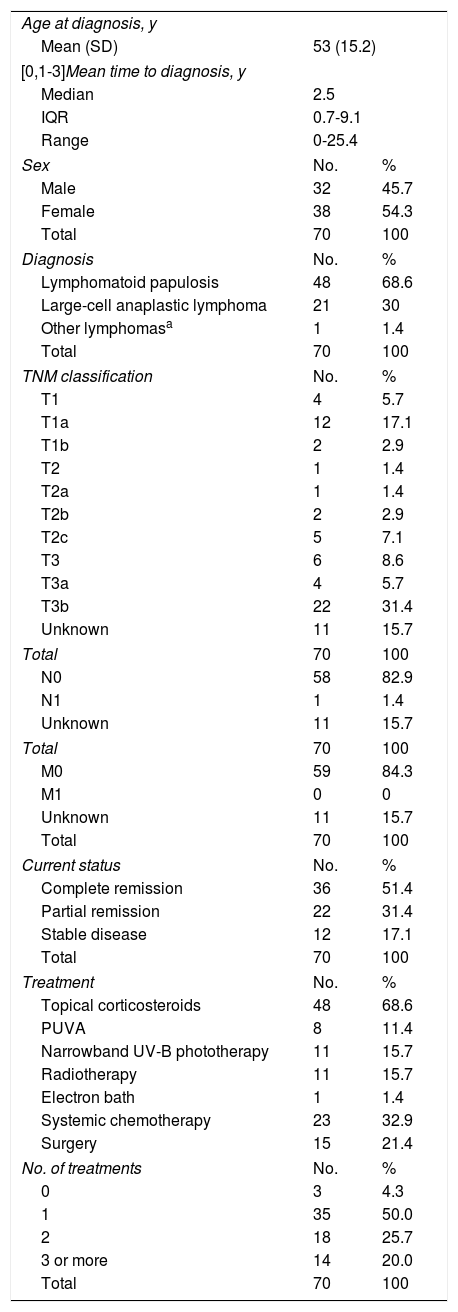
The mean age at diagnosis in the CD30+ CLPD group was 53 (15.2) years, with a mean time to diagnosis of 2.5 years (IQR, 0.7-9.1) and a male to female ratio of 1.2:1. The most common diagnosis was lymphomatoid papulosis, which accounted for 68.6% of all cases. A majority of patients (31.4%) had stage T3b disease. Half of the patients were in complete remission at the time of the last visit. The most common treatments were topical corticosteroids (68.6%) and systemic chemotherapy (32.9%). Half of the patients required just 1 treatment while 25.7% required 2. The characteristics of the patients are summarized in Table 2.
Clinical Characteristics and Treatment of Patients With CD30+ Cutaneous Lymphoproliferative Disorder.
| Age at diagnosis, y | ||
| Mean (SD) | 53 (15.2) | |
| [0,1-3]Mean time to diagnosis, y | ||
| Median | 2.5 | |
| IQR | 0.7-9.1 | |
| Range | 0-25.4 | |
| Sex | No. | % |
| Male | 32 | 45.7 |
| Female | 38 | 54.3 |
| Total | 70 | 100 |
| Diagnosis | No. | % |
| Lymphomatoid papulosis | 48 | 68.6 |
| Large-cell anaplastic lymphoma | 21 | 30 |
| Other lymphomasa | 1 | 1.4 |
| Total | 70 | 100 |
| TNM classification | No. | % |
| T1 | 4 | 5.7 |
| T1a | 12 | 17.1 |
| T1b | 2 | 2.9 |
| T2 | 1 | 1.4 |
| T2a | 1 | 1.4 |
| T2b | 2 | 2.9 |
| T2c | 5 | 7.1 |
| T3 | 6 | 8.6 |
| T3a | 4 | 5.7 |
| T3b | 22 | 31.4 |
| Unknown | 11 | 15.7 |
| Total | 70 | 100 |
| N0 | 58 | 82.9 |
| N1 | 1 | 1.4 |
| Unknown | 11 | 15.7 |
| Total | 70 | 100 |
| M0 | 59 | 84.3 |
| M1 | 0 | 0 |
| Unknown | 11 | 15.7 |
| Total | 70 | 100 |
| Current status | No. | % |
| Complete remission | 36 | 51.4 |
| Partial remission | 22 | 31.4 |
| Stable disease | 12 | 17.1 |
| Total | 70 | 100 |
| Treatment | No. | % |
| Topical corticosteroids | 48 | 68.6 |
| PUVA | 8 | 11.4 |
| Narrowband UV-B phototherapy | 11 | 15.7 |
| Radiotherapy | 11 | 15.7 |
| Electron bath | 1 | 1.4 |
| Systemic chemotherapy | 23 | 32.9 |
| Surgery | 15 | 21.4 |
| No. of treatments | No. | % |
| 0 | 3 | 4.3 |
| 1 | 35 | 50.0 |
| 2 | 18 | 25.7 |
| 3 or more | 14 | 20.0 |
| Total | 70 | 100 |
Abbreviations: IQR, interquartile range; PUVA, psoralen plus UV-A; TNM, tumor-node-metastasis.
There were 37 patients in the OTCL group. Primary cutaneous small/medium-cell CD4+ T-cell lymphoproliferative disorder was the most common lymphoma (26 patients, 70.3%), followed by panniculitis-like T-cell lymphoma and peripheral T-cell lymphoma, nonspecified (5 patients in both cases). There was 1 case of acral CD8+ T-cell lymphoma.
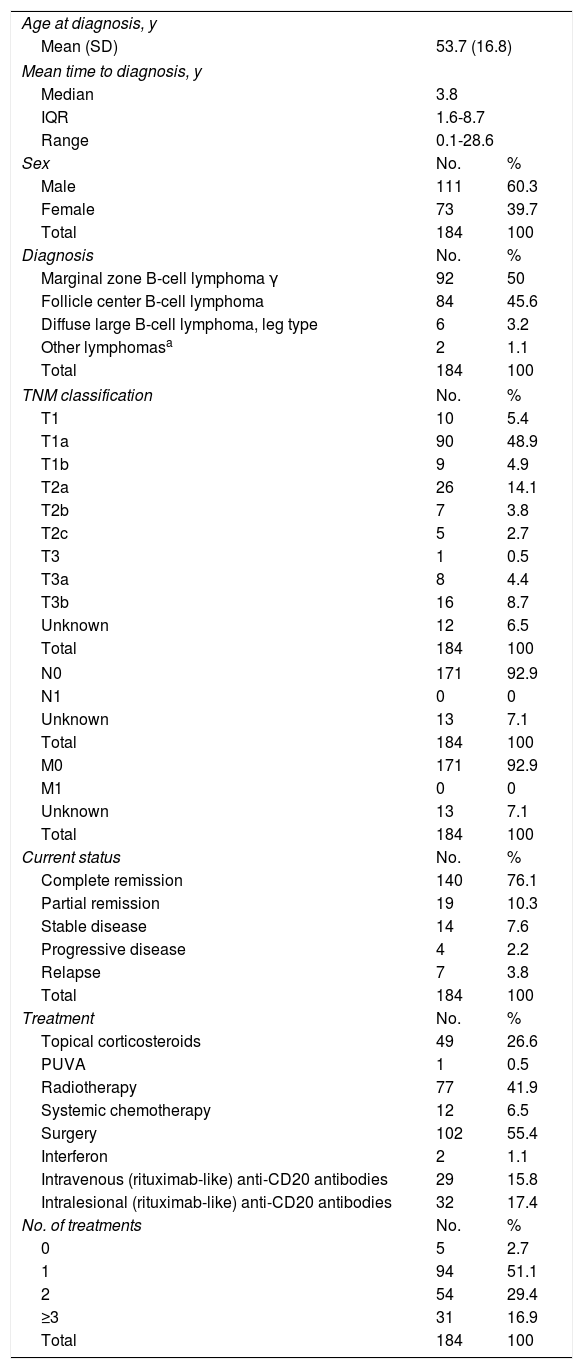
CBCL GroupThe mean age at diagnosis in the CBCL group was 53.7 (16.8) years, with a mean time to diagnosis of 3.8 years (IQR, 0.1-28.6) and a male to female ratio of 1:1.5. The most common diagnoses were primary cutaneous marginal zone lymphoma (MZL) and follicle center B-cell lymphoma (FCL). Practically all the patients had excusive skin involvement and almost half had stage T1a disease. At the time of the last visit, most patients (76.1%) were in complete remission. The most common treatments were surgery (55.4%) and radiotherapy (41.9%). Half of the patients received just 1 treatment while 46.3% required at least 2. The characteristics of the patients are shown in Table 3.
Clinical Characteristics and Treatment of Patients With Primary Cutaneous B-Cell Lymphoma.
| Age at diagnosis, y | ||
| Mean (SD) | 53.7 (16.8) | |
| Mean time to diagnosis, y | ||
| Median | 3.8 | |
| IQR | 1.6-8.7 | |
| Range | 0.1-28.6 | |
| Sex | No. | % |
| Male | 111 | 60.3 |
| Female | 73 | 39.7 |
| Total | 184 | 100 |
| Diagnosis | No. | % |
| Marginal zone B-cell lymphoma γ | 92 | 50 |
| Follicle center B-cell lymphoma | 84 | 45.6 |
| Diffuse large B-cell lymphoma, leg type | 6 | 3.2 |
| Other lymphomasa | 2 | 1.1 |
| Total | 184 | 100 |
| TNM classification | No. | % |
| T1 | 10 | 5.4 |
| T1a | 90 | 48.9 |
| T1b | 9 | 4.9 |
| T2a | 26 | 14.1 |
| T2b | 7 | 3.8 |
| T2c | 5 | 2.7 |
| T3 | 1 | 0.5 |
| T3a | 8 | 4.4 |
| T3b | 16 | 8.7 |
| Unknown | 12 | 6.5 |
| Total | 184 | 100 |
| N0 | 171 | 92.9 |
| N1 | 0 | 0 |
| Unknown | 13 | 7.1 |
| Total | 184 | 100 |
| M0 | 171 | 92.9 |
| M1 | 0 | 0 |
| Unknown | 13 | 7.1 |
| Total | 184 | 100 |
| Current status | No. | % |
| Complete remission | 140 | 76.1 |
| Partial remission | 19 | 10.3 |
| Stable disease | 14 | 7.6 |
| Progressive disease | 4 | 2.2 |
| Relapse | 7 | 3.8 |
| Total | 184 | 100 |
| Treatment | No. | % |
| Topical corticosteroids | 49 | 26.6 |
| PUVA | 1 | 0.5 |
| Radiotherapy | 77 | 41.9 |
| Systemic chemotherapy | 12 | 6.5 |
| Surgery | 102 | 55.4 |
| Interferon | 2 | 1.1 |
| Intravenous (rituximab-like) anti-CD20 antibodies | 29 | 15.8 |
| Intralesional (rituximab-like) anti-CD20 antibodies | 32 | 17.4 |
| No. of treatments | No. | % |
| 0 | 5 | 2.7 |
| 1 | 94 | 51.1 |
| 2 | 54 | 29.4 |
| ≥3 | 31 | 16.9 |
| Total | 184 | 100 |
Abbreviations: IQR, interquartile range; PUVA, psoralen plus UV-B; TNM, tumor-node-metastasis.
γ: Not currently considered an independent entity by the World Health Organization.
Source: Adapted from Swerdlow et al.1
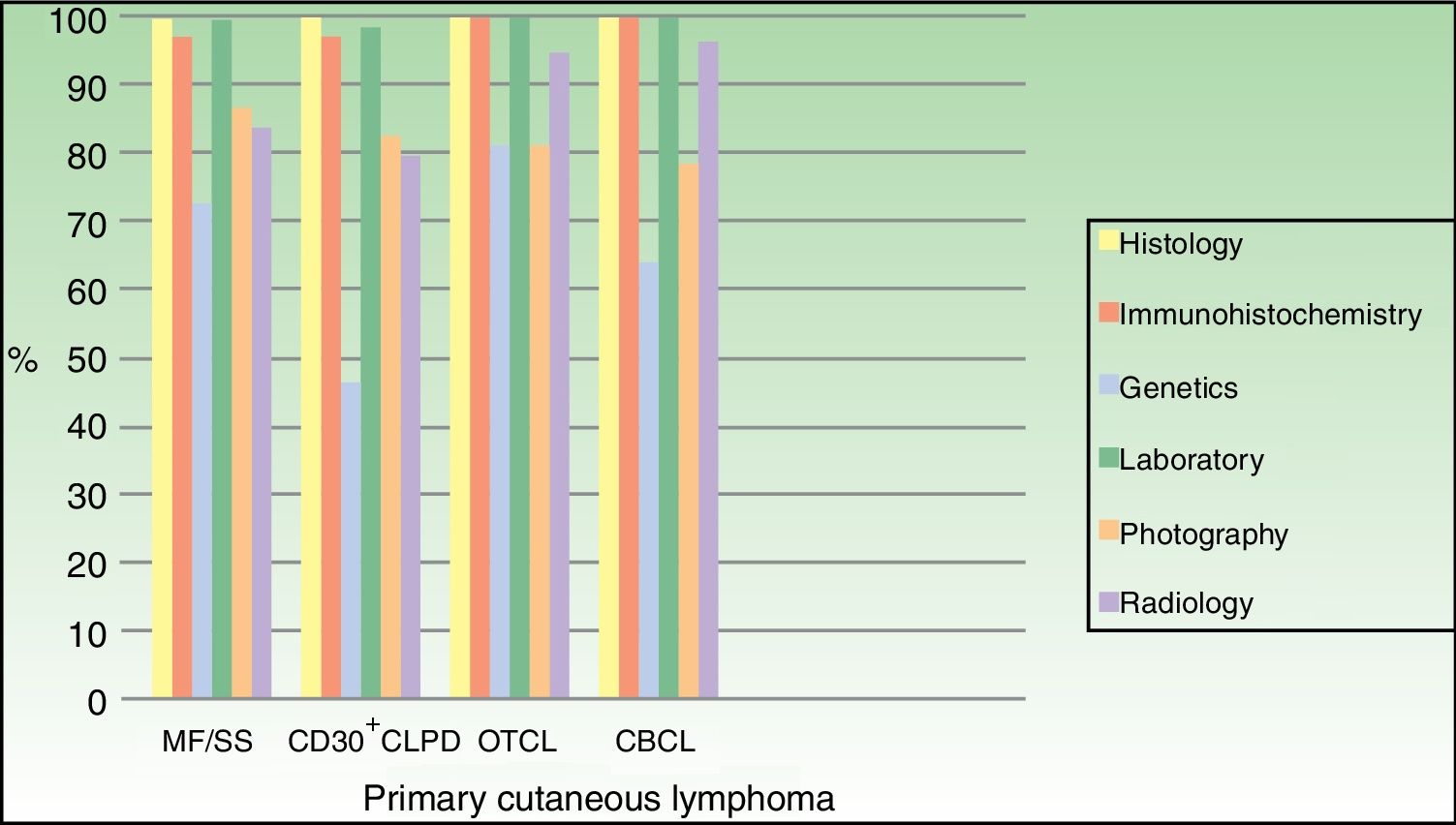
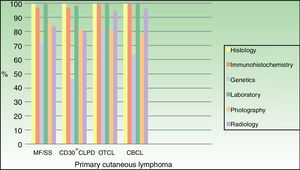
The diagnostic workup for the vast majority of patients included histology, immunohistochemistry, and blood tests. A molecular analysis was performed in 72.7% of patients in the MF/SS group, 46.4% of those in the CD30+ CLPD group, 81% of those in the OTCL group, and 64.1% of those in the CBCL group (Fig. 1).
DiscussionPrimary cutaneous lymphomas are rare, with an estimated incidence of just 1 case per 100 000 population a year.5 Data from Germany,6 the United Kingdom,7 Norway,8 and Denmark9 show an annual incidence of between 2.9 and 4 cases×10.6
It is crucial to create multicenter registries for uncommon conditions such as primary cutaneous lymphoma to facilitate a greater understanding of these diseases and enable clinical studies.
Studies to date by the AEDV's Working Group on Cutaneous Lymphomas have been based on individual databases managed by the different hospitals.10–12 The AEDV's Primary Cutaneous Lymphoma Database will facilitate collaborative research. In its first year of operation, it has already exceeded initial expectations, with 16 hospitals registering data for 638 patients.
For the descriptive analysis in this article, we divided the patients into 4 large groups: MF/SS, CD30+ CLPD, OTCL, and CBCL.
The overall breakdown is similar to that described in the literature,13 with a 75% to 25% split between T-cell and B-cell lymphomas, respectively. MF was the most common diagnosis (55% of all cases), followed by B-cell lymphomas (MZL and FCL) (29%) and CD30+ CLPD (11%), in particular lymphomatoid papulosis.
The profile of patients with MF was also as expected, with a mean age at diagnosis of 55 years, a male to female ratio of 1:1.5, and a median time to diagnosis of 5 years. In accordance with data for European series,13 most patients were diagnosed at an early stage of disease and received treatments specifically targeting the skin. Contrasting information has been described in some US series,14 where over 30% of patients had advanced disease at diagnosis. The different rates are probably due to differences in access to health care. The fact that most patients were in partial remission and had stable disease can be explained by the chronic nature of the disease and the higher proportion of patients with early-stage disease. This observation is also supported by the use of more than 2 treatments in most patients.
The profile of patients with CBCL in the AEDV's registry is also similar to that described in the literature, with a mean age at diagnosis of 54 years and a slight male predominance.7 We did not observe any substantial differences between the proportion of patients with MZL and FCL, despite earlier reports of a dominance of FCL.13 This lack of significant differences has already been reported for countries such as Austria.6 The similar proportion of MZL and FCL cases in Spain may have several explanations, including the low prevalence of etiologic factors, such as Borrelia burgdorferi, diagnostic refinement in cases of MZL with reactive follicle centers, and a lack of specific markers.
Most patients had just a single lesion measuring less than 5cm in diameter and, in line with the recommendations of the EORTC and the ISCL, largely received just 1 treatment (surgery or radiotherapy).15
The characteristics of the CD30+ CLPD group are also similar to those described in the literature.13,16,17 This variant was the second most common type of T-cell lymphoma in the group (29%) and lymphomatoid papulosis was more common than anaplastic large-cell T-cell lymphoma.
According to the data on tests performed, gene rearrangement analyses were not performed in 30% of patients with T-cell lymphoma and 36% of those with B-cell lymphoma. While these tests are not always essential for diagnosis, they are advisable in most patients with clinically suspected disease. Not all hospitals will have the equipment needed to run these tests, of course, and we know from our experience that many solitary lesions (mostly B-cell lymphomas or small/medium CD4+ lymphoproliferative disorders) are suspected to be another entity prior to excision. In the case of lymphomatoid papulosis, lesions can mimic bite reactions or lichenoid pityriasis.
One of the limitations of the registry is that not all diagnoses may be accurate. There are plans to set up a committee of experts to review complicated cases prior to the publication of studies about specific cases from the registry. We know that primary cutaneous lymphomas are not always easy to diagnosis. Examples are incipient MF, which requires the application of accurate diagnostic criteria18 and cases of overlap between MZF and FCL or lymphomatoid papulosis and CD30+ large-cell anaplastic lymphoma.
In many hospitals, patients with severe disease are managed by hematologists, meaning that they are seen less frequently by dermatologists. This is an important consideration as it could influence type of remission and treatment. As a final limitation, because the registry contains patients seen at tertiary-care hospitals, it may not be fully representative.22 Notwithstanding, risk of selection bias should be limited by the large number of participating hospitals and the consecutive inclusion of all cases.
ConclusionThis is the first study to describe the clinical characteristics of patients with primary cutaneous lymphoma in Spain and the results are similar to those described in the literature.
Our findings show that the AEDV'S Primary Cutaneous Lymphomas Registry is serving its purpose. The results from this year are very encouraging as they herald the creation of a good database that will facilitate the performance of retrospective and prospective epidemiological studies and ultimately contribute to improving our understanding of primary cutaneous lymphoma. Now that the project is underway, we need to ensure that it continues and to lay the groundwork for studies of different entities that will shed light on behavior, management, and prognosis.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Peñate Y, Servitje O, Machan S, Fernández-de-Misa R, Estrach MT, Acebo E, et al. Registro de linfomas cutáneos primarios de la AEDV: primer año de funcionamiento. Actas Dermosifiliogr. 2018;109:610–616.