Hepatitis C virus (HCV) infection is common, with an incidence of 3% in the general population. It can present as acute hepatitis or, more frequently, as chronic asymptomatic hepatitis, which, over years, can progress to more severe disease, such as cirrhosis of the liver or hepatocellular carcinoma. Until recently, the treatment of chronic hepatitis C infection has been based on the combined use of pegylated interferon alfa-2a or 2b plus ribavirin, achieving a persistent virological response in less than 50% of patients with genotype-1 infection. This has made it necessary to develop new treatments, one of which is telaprevir (Incivo), a novel drug whose main dose-limiting side effect is skin toxicity. In order to make dermatologists aware of the existence of this new drug, we present the case of a 58-year-old woman treated with telaprevir.
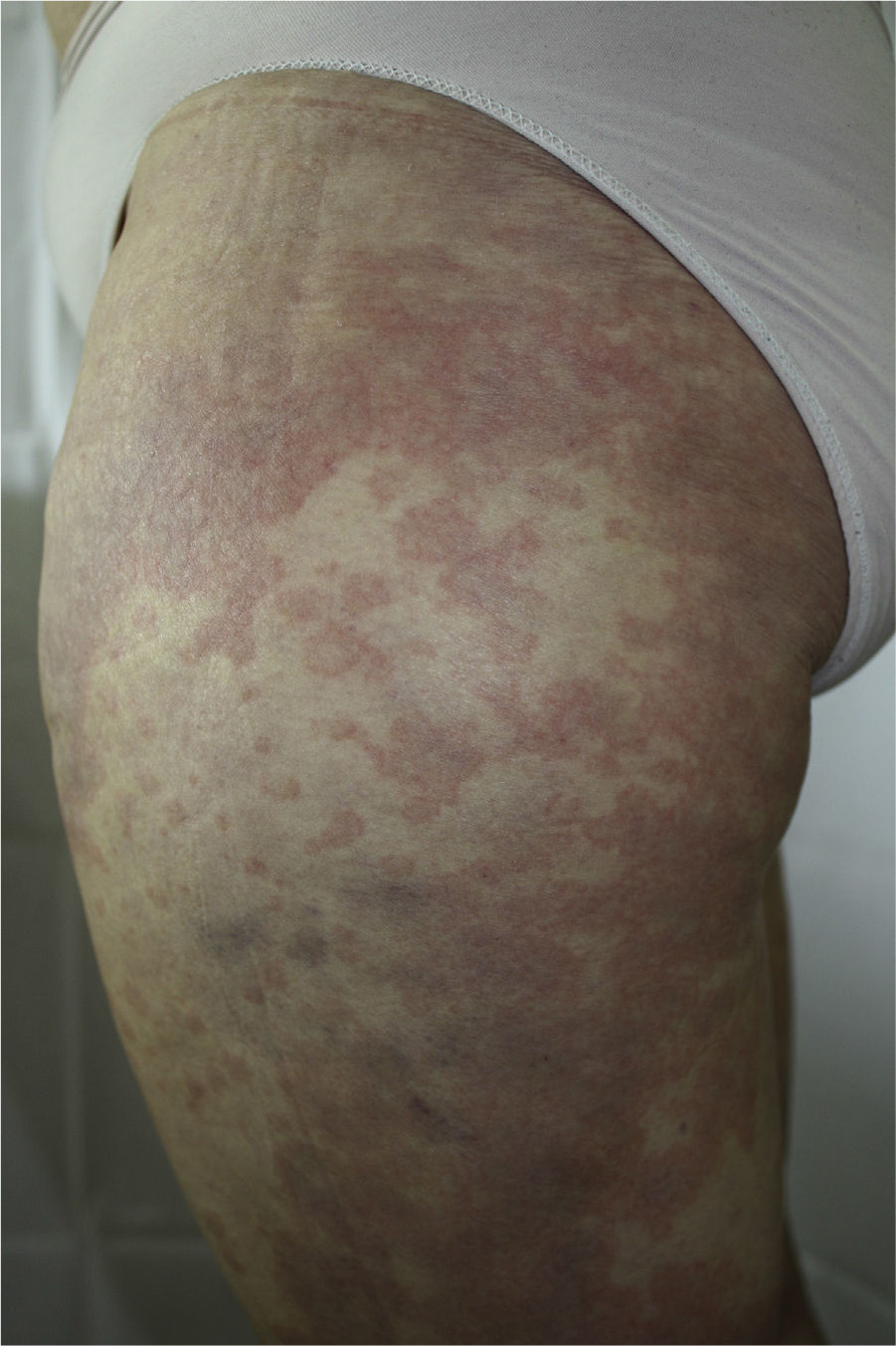
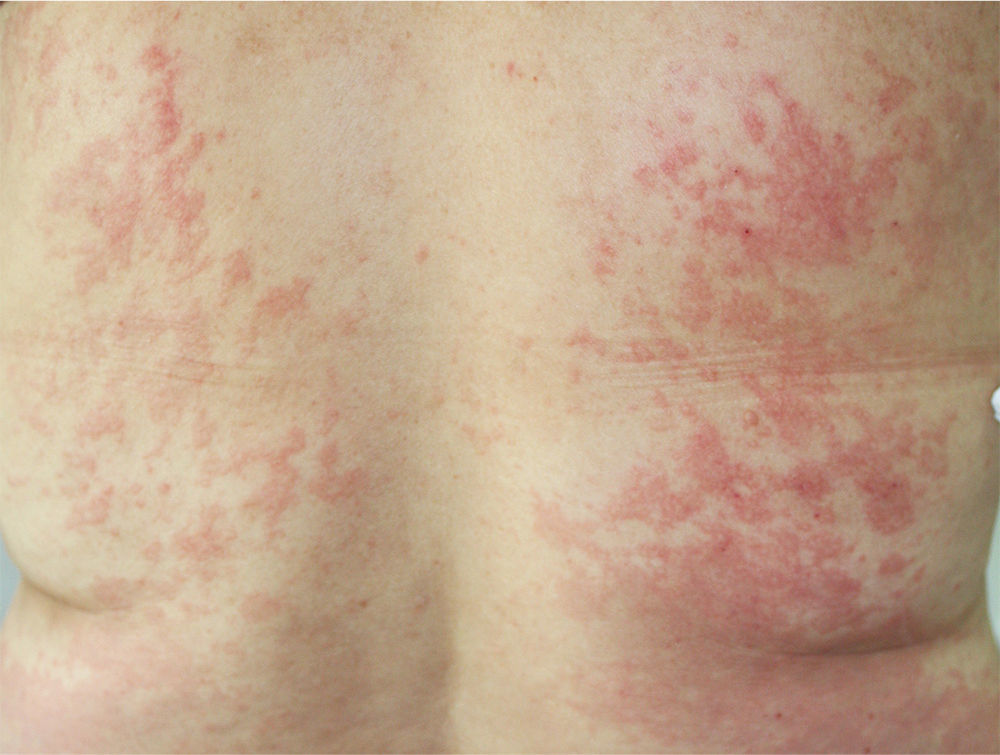
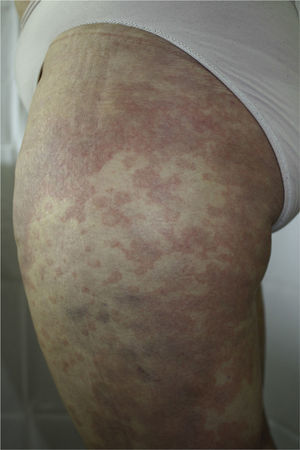
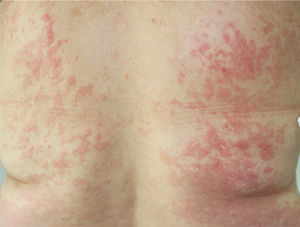
The patient had been diagnosed with chronic HCV genotype 1 infection, but had no other past medical history of interest. She had previously been treated with peginterferon and ribavirin, achieving a transitory response with subsequent relapse. It was decided to start triple therapy by adding telaprevir to the aforementioned drugs, achieving an undetectable viral load in week 3 of treatment. She was seen in dermatology outpatients in week 4 of treatment for the appearance of persistent, pruritic skin lesions that had started to appear 4 days earlier. Physical examination revealed an urticarial rash (Fig. 1) with an annular morphology that affected around 30% of the body surface area. The rash was located on the proximal third of the lower limbs and on the back (Fig. 2). The lesions blanched under pressure and the mucosas were not affected. Topical therapy with betamethasone 17-valerate was prescribed and weekly follow-up was performed until the end of treatment. During this period the lesions showed no progression and they resolved 2 weeks after completing the course of telaprevir.
Knowledge of the replication cycle of HCV and characterization of the viral enzymes has led to the identification of new therapeutic targets that inhibit these enzymes. Telaprevir is an NS3/4A protease inhibitor approved by the Food and Drug Administration and the European Medicines Agency for the treatment of chronic HCV infection in naïve patients and in those previously treated with interferon and ribavirin for genotype 1 infection. The introduction of this drug has led to an increase in the rate of persistent viral response, and treatment time can occasionally be shortened.1 However, the most important limitation to treatment comes from the drug's adverse skin effects. The recommended treatment regimen includes 12 weeks of triple therapy (telaprevir, peginterferon, and ribavirin) followed by a further 12 to 36 weeks of treatment with peginterferon plus ribavirin.2 In the phase II and phase III placebo-controlled studies, the incidence of skin reactions during the 12-week treatment period with telaprevir was 56%, in comparison with 34% observed in patients treated with placebo and peginterferon alfa/ribavirin.3 The skin reactions included local reactions at the site of injection and pruritic maculopapular rashes on the trunk and limbs; the reactions were usually well tolerated and showed a low probability of progression to more severe disorders.4,5
The management and treatment of the skin reaction are based on the severity of the lesions and on the presence of systemic symptoms and abnormalities in the blood tests. Grade I, or mild, is defined as a localized or limited skin rash with no systemic signs and with no mucosal involvement. Grade II, or moderate, is a reaction that affects a maximum of 50% of the body surface area and that causes no epidermal detachment. Mucosal inflammation may be present but there are no ulcers or systemic symptoms such as fever and joint pain, and no eosinophilia. Grade III, or severe, is a reaction in which the skin lesions affect more than 50% of the body surface area, or a lower percentage if any of the following characteristics are observed: presence of vesicles or bullae, mucosal ulcers, epidermal detachment, target lesions, palpable purpura, or erythema that does not blanch under pressure. Finally, grade IV, or life-threatening, is defined as the presence of acute generalized exanthematous pustulosis, a delayed drug hypersensitivity syndrome (DRESS), toxic epidermal necrolysis, or Stevens-Johnson syndrome.5,6
In patients, 90% of these reactions were grade I or II, characterized by pruritic eczematous lesions that affected less than 30% of the body surface area. Most reactions occurred during the first 4 weeks of treatment. Only a small percentage of patients treated with telaprevir developed serious skin reactions of grade III or IV.6 Treatment interruption is not required for grade I or grade II reactions; in these patients, periodic follow-up should be performed until complete resolution of the reaction because of its possible progression to a severe skin reaction.7 Treatment with telaprevir should only be interrupted for reactions of grade III or IV, with the subsequent sequential interruption of ribavirin and interferon if no improvement is observed over the following 7 days. Telaprevir should never be reintroduced.8 The skin lesions can be treated with topical corticosteroids, but an association with systemic corticosteroids can produced a loss of efficacy of telaprevir and can modify its serum levels due to an interaction through both the CYP3A4 and the glycoprotein-P pathways.20 For the same reason, the simultaneous use of telaprevir with other drugs, such as astemizole and terfenadine, is also contraindicated.9 The fecal excretion of telaprevir after it undergoes hepatic metabolism is probably the cause of anorectal symptoms such as pruritus.
In conclusion, we have presented a skin reaction to a new drug for the treatment of HCV infection. Management of this reaction requires dermatologic follow-up in order to optimize treatment.
Please cite this article as: López-Villaescusa M, Pérez-García L, Rodríguez-Vázquez M, Martínez-Martínez M. Toxicidad cutánea por telaprevir: un nuevo fármaco que es necesario conocer. Actas Dermosifiliogr. 2014. 105:315–317.