Sentinel lymph node biopsy is performed routinely in melanoma because lymph node progression has been shown to be the strongest predictor of survival. Given the proven relevance of biopsy findings in this type of skin cancer, the procedure has been extended to other skin tumors. Experience in nonmelanoma cancer is much more limited and the prognostic usefulness of biopsy results remains to be shown. This critical review of the literature on the various skin tumors in which sentinal node biopsy has been practiced discusses the usefulness of this procedure.
La biopsia del ganglio centinela es una técnica que se realiza de forma rutinaria en el manejo del melanoma, en el cual la afectación ganglionar ha demostrado ser el mejor indicador pronóstico de supervivencia. Dado que la utilidad de la misma en este tipo de neoplasia cutánea está demostrada, se ha venido realizando en otros tumores cutáneos en los cuales la experiencia es mucho menor y cuya utilidad en la mayoría de los casos está todavía por demostrar. Realizamos una revisión crítica de los distintos tumores cutáneos en los cuales se ha realizado esta técnica y se discute acerca de su utilidad.
Sentinel lymph node biopsy is based on the hypothesis that efferent lymphatic flow from solid tumors is not random but rather follows a pattern in which there is spread to an initial node. From there, secondary spread occurs to other nodes. If this first lymph node is identified and selectively biopsied, a direct histological assessment of lymphatic spread is possible. In contrast, negative pathology findings for this lymph node would preclude unnecessary lymphadenectomies.
The technique has been shown to provide the most accurate prognosis for survival in patients with melanoma.1 Likewise, such an approach means that prophylactic lymphadenectomies can be avoided as these are usually unnecessary.
Mechanism of Lymphatic MetastasisThe cells responsible for lymphatic metastasis reach the lymph nodes via afferent lymphatic vessels in the subcapsular sinusoids, either individually or as small groups of cells. Once the tumor cells reach the subcapsular space, capsule involvement can occur at any time after lymph node invasion.
The tumor cells proliferate, in principle in the cortex. From there, they can spread to the lymph node medulla and replace normal lymph node structure. Tumor cells spread through efferent lymph vessels in the hila of lymph nodes.
The pattern of growth of lymph node metastases has been well established; these begin as isolated tumor cells, then turn into micromestases, before finally becoming macrometastases.
Sentinel Lymph Node TechniqueTracersFor the detection of sentinel lymph nodes, specific tracers are injected intradermally close to the tumor to trace the characteristic drainage pattern for each patient. In general, radioactive isotopes are used (technetium [Tc]) in the form of colloidal particles that may or may not be associated with vital dyes.
After the administration of tracer, increased interstitial pressure drives the particles through the vessels to the draining nodes, where they remain (in what is the sentinel lymph node). Appropriate imaging techniques are then used to detect the tracers.
According to the size of the colloid used to vehiculize the Tc (between 2nm and 400nm), the rate of transport to and residence time in the lymph node will vary. The most widely used radiomarkers include 99mTc-albumin nanocolloid, filtered 99mTc-sulfur colloid, and 99mTc-amonium sulfide. It should be noted that none of these colloidal systems have a uniform particle diameter but rather a range of diameters distributed around a mean value. The most widely used are intermediate colloids, between 5 and 80nm. These include nanocolloidal albumin, colloidal sulfide, and colloidal rhenium sulfide, which all have the advantage of lower systemic penetration and sharply outline the draining nodes.
DetectionPreoperative detection is performed in the following ways:
- 1.
Dynamic scintigraphy, which allows the direction and number of outflow paths to be visualized.
- 2.
Static scintigraphy, which visualizes the final site of the tracer, marking a “hot spot” with dye in the skin.
In recent years, studies have been performed with new tracer systems such as the following2–6:
- 1.
Imaging of indocyanine green (Dianogreen) fluorescence with infrared cameras. A fluorescent tracer is used that can be visualized with cameras set up to detect the wavelength emitted. This is a real-time system.The advantages of this method are that a) it does not depend on γ-ray counters or radioisotope detectors; b) lymph node detection is easier than with radiotracers; c) it is more useful for detecting nodes close to the tumor; and d) neither the patients nor the operators are exposed to ionizing radiation.
The disadvantages of this method are that a) it only detects lymph nodes up to 2cm deep and b) quantitative study of tissue fluorescence is difficult, and so there is a risk of missing sentinel lymph nodes when other larger ones are resected.
- 2.
Gold nanocages detected by photoacoustic systems. These nanocages are readily conjugated to antibodies for specific receptors.4
The histopathology of resected nodes involves examining multiple slices from the entire node, taken from a central section along the main axis of the node (sections of 200μm every 2mm)
Hematoxylin-eosin staining should be used; immunohistochemical (IHC) techniques are then used when hematoxylin-eosin staining is negative. Immunohistochemical reagents used in the case of melanoma are S-100, MELAN-A, and HMB-45.
Indications for the TechniqueTo date, this approach has been shown to provide useful prognostic information when lymph node spread is a concern in a wide range of solid tumors, such as melanoma,7 breast cancer,8 cancer of the penis9 and vulva,10 thyroid carcinoma,11 lung cancer,12 colorectal cancer,13 prostate cancer,14 and gastrointestinal cancer.15
Sentinel Lymph Nodes in Nonmelanoma Skin CancerIn addition to the established indications, there are a number of nonmelanoma skin cancers for which the possible benefit of this technique is under investigation.
At present, sufficiently large studies have not been performed to provide clear indications.
Sentinel Lymph Node Biopsy in Squamous Cell Carcinoma of the SkinSquamous cell carcinoma of the skin accounts for approximately 20% of skin cancers and is the second most common form of skin cancer in whites.16–18
This type of tumor can grow until it forms a large mass and becomes invasive. Unlike basal cell carcinomas, squamous cell carcinomas spread readily to regional and distant lymph nodes. The overall rate of metastasis is 5% and the 5-year survival after metastasis is 26%.18
Metastases usually occur within 2 years of diagnosis (80%)16,19–21; however there have been reports of later metastasis.22
The essential points to analyze here are what we consider high-risk squamous cell carcinoma of the skin and how we manage these patients if there is no clinical or radiological evidence of lymph node involvement (N0).
- 1.
We understand high-risk squamous cell carcinoma of the skin as a primary tumor with a risk of metastasis of greater than 5%, where the risk is assessed according to unfavorable patient-related factors (for example, immunosuppression) or tumor-related factors.23
Several studies have assessed these factors.16,24,25 However, we believe it important to highlight the study by Breuninger et al,25 which included 615 patients who underwent surgery for squamous cell carcinoma of the skin. The multivariate analysis performed included tumor thickness, size, site, degree of histologic differentiation, desmoplastic histologic subtype, history of multiple squamous cell carcinomas, and immunosuppression.
The primary objective of the study was to determine the moment in which metastases occurred and/or the time to local recurrence (defined as the time from tumor diagnosis to diagnosis of the metastasis or recurrence). The mean duration of follow-up was 43 months.
Metastasis occurred in 26 (4%) of the 615 patients, and local recurrence occurred in 20 (3%). No patient presented with metastasis after more than 4 years.
The authors reported that the squamous cell carcinomas of the skin less than 2mm thick did not metastasize, those 2-6mm thick were associated with an intermediate risk of metastasis (4%), and those more than 6mm thick had a high risk of metastasis (16%). The authors concluded that the current TNM staging system for squamous cell carcinoma of the skin should be revised, and tumor thickness included in the classification.
- 2.
Regarding the management of high-risk squamous cell carcinoma of the skin without clinical or radiological evidence of lymph node involvement (N0), the problem is that, to date, there are no universally accepted guidelines. The alternative approaches available are a) close monitoring, b) adjuvant postoperative radiotherapy, c) prophylactic regional lymphadenectomy, and d) lymphadenectomy with or without radiation therapy.
In recent years, several studies have been carried out to assess the usefulness of sentinel lymph node biopsy in these patients. A review of the studies performed to date highlights the difficulty of interpreting the results and the problems in drawing conclusions, as squamous cell carcinomas of the skin at different sites are often mixed (for example, squamous cell carcinomas on the face with carcinomas on the tongue).
Most of the studies are performed by ear-nose-throat specialists, plastic surgeons, and maxillofacial surgeons. We did not find any studies that assessed the usefulness of this technique in exclusively cutaneous tumors on the face, for example.
The Clinical Practice Guidelines in Oncology (issued by the National Comprehensive Cancer Network [NCCN])26 state that that sentinel lymph node biopsy should be considered in certain high-risk tumors, although the benefit of this technique has not been confirmed.The technique has been practiced for years for squamous cell carcinoma of the oral cavity, oropharynx, and genitals, and the results of several studies have suggested that this approach to the management of these tumors is promising, given the high sensitivity for detection of lymph node metastases.27–30
The advantages of sentinel lymph node biopsy in this group of patients are as follows:
- 1.
The biopsy provides information on the staging of squamous cell carcinoma, identifying the draining nodes and subclinical metastases.
The superficial lymphatic system of the head and neck is characterized by the unpredictability of the drainage paths. Previous studies have shown that performing elective lymphadenectomy, without lymphoscintigraphy prior to the operation, could lead to inappropriate target node dissection in up to half the cases.
- 2.
The biopsy provides prognostic information.
- 3.
Biopsy findings help in subsequent decision making: lymphadenectomy or administration of radiotherapy or chemotherapy in patients with positive lymph node biopsy.
- 4.
The morbidity associated with radiotherapy or prophylactic lymphadenectomy (secondary lymphedema and neural damage) is avoided.
- 5.
The findings might have repercussions for the survival of these patients, given that mapping and early treatment of metastasis could enable a more targeted therapeutic approach, applied in theory in earlier stages of the disease.
Some authors indicate that it is possible to have greater control over metastatic disease in squamous cell carcinoma than in melanoma due to the greater propensity of squamous cell carcinoma for orderly dissemination and its greater sensitivity to radiotherapy.31 On the other hand, resection of micrometastases themselves could be beneficial in terms of survival.
We believe it is of interest to highlight the following studies, as they are the ones that included the largest numbers of patients. Renzi et al32 performed sentinel lymph node biopsy in 22 patients with high-grade N0 squamous cell carcinoma of the skin. The authors defined high-grade squamous cell carcinoma as tumors with one of the following characteristics: size greater than 2cm; site on the lip or in front of the ear; local recurrence or recurrence over previously damaged skin; poor histologic differentiation; perineural invasion; depth greater than 4mm; involvement of the reticular dermis or subcutaneous fat; involvement of bone, muscle, or cartilage; and immunosuppression. Of the 22 patients studied, positive sentinel lymph nodes were detected in just 1 (4.5%). This patient had local recurrence of the disease, whereas the remaining patients with negative sentinel lymph node did not have recurrence during follow-up (mean of 17 months). The authors recognize the limitations of the study. These include the small number of patients, the duration of follow-up, and the lack of uniform criteria for including patients with high-risk squamous cell carcinoma.
Reschly et al31 resected or biopsied the sentinel lymph node of 9 patients with squamous cell carcinoma of the skin located on the face or limbs. They considered high-risk tumors as those that met at least one of the following 9 criteria: certain types of treatment used, recurrent tumor (previous treatments), size greater than 2cm, site on the lip or in front of the ear, deep invasion, poor histologic differentiation, histologic evidence of perineural invasion, precipitating factors other than UV radiation, and immunosuppression.
A positive sentinel lymph node was found in 4 patients (44%). Two of the 4 patients with positive sentinel lymph node biopsy died of metastatic disease within 2 years. The 5 patients with negative sentinel lymph node biopsy were alive with no evidence of recurrent disease after 8 months of follow-up.
The rates of positive lymph node biopsy reported by these authors are as high as those reported for high-risk squamous cell carcinoma of the genitals or oropharyngeal cavity. This could be due to the low number of patients and the lack of uniform criteria for defining high-risk squamous cell carcinoma. The authors concluded that this technique is useful for the management of these patients.
Conclusion for Squamous Cell Carcinoma of the SkinAlthough the studies performed to date have included low numbers of patients, most suggest that this technique could be useful in high-risk squamous cell carcinoma. Patients with positive sentinel lymph node biopsy can be given more aggressive treatments and follow-up protocols, which could translate into longer survival.
Morbidity would be decreased in patients with negative sentinel lymph node biopsy as more aggressive and unnecessary treatments would be avoided.
The main difficulty comes when establishing the indication, that is, when we have to define what constitutes high risk, given the different criteria used in the studies. The study by Brantsch et al25 could be used as a starting point, with subsequent studies designed with unified criteria.
With the data currently available, sentinel lymph node biopsy seems appropriate in squamous cell carcinoma with a risk of metastasis of greater than 5%, which in the studies reviewed is when the squamous cell carcinoma has a depth greater than 6mm.
We also believe that it is important not to lose sight of the life context of our patients. They are usually very old, and performing these types of techniques would not make sense in terms of risk-benefit.
Likewise, we should take into account the erratic nature of lymphatic drainage in the head (a common site for squamous cell carcinoma of the skin), and this could also limit the usefulness of the technique.
Sentinel Lymph Node Biopsy in Merkel Cell CarcinomaMerkel cell carcinoma is a rare, clinically aggressive neuroendocrine tumor that tends to spread locally and metastasize at a distance.This tumor affects elderly people, and its incidence is higher in immunosuppressed patients, HIV-positive patients, and organ transplant recipients. More recently, the etiology of this tumor has been linked to the Merkel cell polyomavirus, which is found integrated into the cellular genome in more than 80% of the cases.33–35
The incidence is on the rise, possibly because of more frequent detection in clinical practice, an increase in the population older than 70 years, and the use of cytokeratin 20, a very specific marker for Merkel cell carcinoma.36
This tumor occurs much more frequently in whites (93% of the cases), and is rarely reported in blacks. Slightly more are diagnosed in men than in women (a ratio of between 2 and 3 to 1).
The most frequent site is the head and neck (essentially in the periocular and perioral area), followed by the limbs, and, more rarely, the trunk.
The tumor presents as nodular, erythematous, bluish lesions. Telangiectasias may be apparent on its surface, and so it is often confused with basal cell carcinoma. Tumor sizes vary between 0.2 and 20cm, although most are less than 2cm in diameter. At the time of diagnosis, lymph node involvement is observed in 15% to 60% of the patients, and distant metastases in 1% to 6% of the patients. Immunohistochemical staining can help in the differential diagnosis of the tumor. Typically, staining is positive for cytokeratin 20 and negative for thyroid transcription factor 1 (ruling out small cell lung cancers). Other markers of diagnostic utility are specific neuronal enolase, chromogranin, and synaptophysin, among others.37
Staging in Merkel Cell CarcinomaNo staging system has been universally accepted; in fact, until 2009, 5 different staging systems were being used to describe Merkel cell carcinoma. For this reason, the American Joint Committee on Cancer established a new staging system based on consensus38 (Table 1).
Current Staging in Merkel Cell Carcinoma.
| Primary Tumor (T) | |
| TX | Nonevaluable primary tumor |
| T0 | Evidence of a primary tumor (for example, lymphatic or metastatic presentation without associated primary tumor) |
| Tis | In situ primary tumor |
| T1 | Maximum tumor size: ≤ 2 cm |
| T2 | Maximum tumor size: > 2cm, but ≤ 5cm |
| T3 | Maximum tumor size: > 5 cm |
| T4 | Bone, muscle, fascia, or cartilage invasion by primary tumor |
| Regional Lymph Nodes (N) | |
| NX | Nonevaluable regional lymph nodes |
| N0 | No metastasis in regional lymph nodes |
| cN0 | Negative lymph nodes in clinical examinationa (pathology study not performed) |
| pN0 | Negative lymph nodes in pathology study |
| N1 | Metastases in regional lymph node(s) |
| N1a | Micrometastasesb |
| N1b | Macrometastasesc |
| N2 | In transit metastasesd |
| Distant Metastasis (M) | |
| M0 | No distant metastasis |
| M1 | Metastases beyond regional lymph nodes |
| M1a | Metastases to the skin, subcutaneous tissue, or distant lymph nodes |
| M1b | Lung metastases |
| M1c | Metastases to other visceral sites |
Taken from AJCC: Merkel cell carcinoma.38
Clinical staging is considered the most important factor; thus, survival at 5 years is 81% for stage Ia, 67% for stage Ib, 52% for stage II, and 11% at 2 years for stage III.39
Recurrence rates between 40% and 45% have been reported, and can be as high as 77% when tumors are located on the head and neck. The mean time to recurrence is 8 months, and 90% recur within 2 years.40
Patients with Merkel cell carcinoma should undergo detailed study that includes a search for satellite skin lesions, lymph node palpation, and computed tomography, although Sheela et al41 concluded in their review that computed tomography has poor sensitivity for detecting lymph node involvement and a high specificity for detecting distant metastases.
In fact, when tumor involvement is visible in computed tomography, the chance of curative treatment vanishes. However, several recent studies discuss the use of positron emission tomography combined with computed tomography in the follow-up and subsequent management of patients with this diagnosis.42
Lesion size greater than 2cm and the presence of local recurrence or distant metastases are considered factors for poor prognosis. Some authors have also found that histology showing small or intermediate cells with a high mitotic index can also be considered as predictors of poor prognosis. Other authors, however, have found no prognostic value in histologic type (solid, trabecular, or diffuse) or in cell size (small, medium, or large).43,44 The most important prognostic factor at present is the presence of lymph node involvement, making sentinel lymph node biopsy a valuable diagnostic technique.45
Sentinel Lymph Node Biopsy in Merkel Cell CarcinomaThe presence of lymph node involvement and the presence of distant metastases are the prognostic factors associated with the poorest survival rates in Merkel cell carcinoma. The need for prophylactic regional lymphadenectomy is being debated in view of the associated morbidity and the lack of improvement in survival.
Complete lymph node dissection has been proposed for large tumors, those on the head and neck, those with a small-cell histology, and those with lymphovascular invasion.36
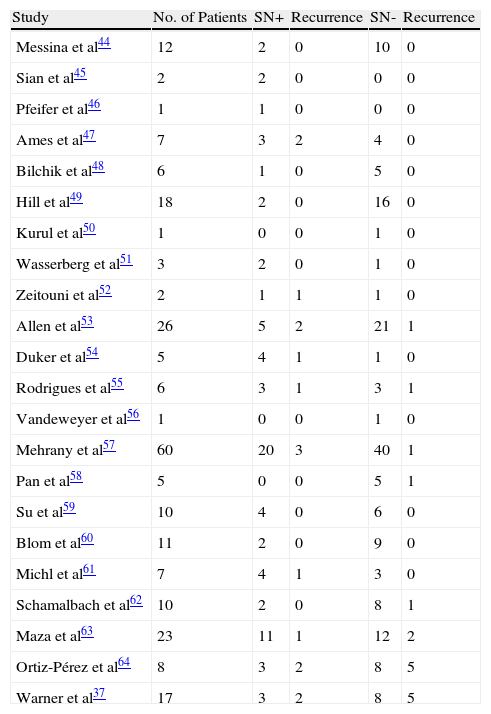
Performing sentinel lymph node biopsy is an attractive alternative to elective complete lymphadenectomy in that it allows more appropriate selection of candidates and also early detection of lymph node involvement.The contribution of sentinel lymph node biopsy in Merkel cell carcinoma is generally accepted.46–49Table 2 summarizes the studies conducted on this topic to date.37,45–64
Studies Conducted to Date on Sentinel Lymph Node Biopsy in Merkel Cell Carcinoma.a
| Study | No. of Patients | SN+ | Recurrence | SN- | Recurrence |
| Messina et al44 | 12 | 2 | 0 | 10 | 0 |
| Sian et al45 | 2 | 2 | 0 | 0 | 0 |
| Pfeifer et al46 | 1 | 1 | 0 | 0 | 0 |
| Ames et al47 | 7 | 3 | 2 | 4 | 0 |
| Bilchik et al48 | 6 | 1 | 0 | 5 | 0 |
| Hill et al49 | 18 | 2 | 0 | 16 | 0 |
| Kurul et al50 | 1 | 0 | 0 | 1 | 0 |
| Wasserberg et al51 | 3 | 2 | 0 | 1 | 0 |
| Zeitouni et al52 | 2 | 1 | 1 | 1 | 0 |
| Allen et al53 | 26 | 5 | 2 | 21 | 1 |
| Duker et al54 | 5 | 4 | 1 | 1 | 0 |
| Rodrigues et al55 | 6 | 3 | 1 | 3 | 1 |
| Vandeweyer et al56 | 1 | 0 | 0 | 1 | 0 |
| Mehrany et al57 | 60 | 20 | 3 | 40 | 1 |
| Pan et al58 | 5 | 0 | 0 | 5 | 1 |
| Su et al59 | 10 | 4 | 0 | 6 | 0 |
| Blom et al60 | 11 | 2 | 0 | 9 | 0 |
| Michl et al61 | 7 | 4 | 1 | 3 | 0 |
| Schamalbach et al62 | 10 | 2 | 0 | 8 | 1 |
| Maza et al63 | 23 | 11 | 1 | 12 | 2 |
| Ortiz-Pérez et al64 | 8 | 3 | 2 | 8 | 5 |
| Warner et al37 | 17 | 3 | 2 | 8 | 5 |
Sources: Gupta SG et al,36 Messina JL et al,44 Sian KU et al,45 Pfeifer T et al,46 Ames SE et al,47 Bilchik AJ et al,48 Hill AD et al,49 Kurul S et al,50 Wasserberg N et al,51 Zeitouni NC et al,52 Allen PJ et al,53 Duker I et al,54 Rodrigues LK et al,55 Vandeweyer E et al,56 Mehrany K et al,57 Pan D et al,58 Su LD et al,59 Blom A et al,60 Michl C et al,61 Schmalbach CE et al,62 and Maza S et al.63
Several authors have reached the conclusion that the risk of recurrence and metastatic disease is greater if a sentinel lymph node biopsy is positive, while a negative biopsy is predictive of better short-term survival.36
It is important to note that Merkel cell carcinoma is a rare condition, and so experience with sentinel lymph node biopsy in this setting is necessarily limited. Gupta et al36 described their experience in 61 patients with Merkel cell carcinoma. Thirty of these underwent sentinel lymph node biopsy and they also performed a metaanalysis of a further 92 case reports of patients diagnosed with Merkel cell carcinoma who underwent sentinel lymph node biopsy. Overall, then, 122 patients with Merkel cell carcinoma with no clinical evidence of lymph node spread were studied. Sentinel lymph node biopsy was performed, and found to be positive, in 39 (32%). The authors also found that the incidence of positive sentinel lymph node biopsy increased with increasing primary tumor size such that in patients with Merkel cell carcinoma larger than 2cm, 52% had a positive sentinel lymph node biopsy compared to 29% of patients with a tumor size less than 2cm (although the differences were not statistically significant, P=.23). At 3 years, the recurrence rate in patients with a positive sentinel lymph node biopsy (32%) was 60%, 3 times higher than the rate in patients with a negative sentinel lymph node biopsy (20%). This study demonstrates the high rate of positive sentinel lymph node biopsies in patients with no clinical or radiologic evidence of involvement of lymphatic territories; from a prognostic point of view, the authors found survival benefit in association with a positive lymph node biopsy when patients underwent early lymphadenectomy compared to those who did not undergo the procedure. The authors concluded that sentinel lymph node biopsy should be performed in patients with N0 Merkel cell carcinoma to establish prognosis and determine treatment.
In the largest series published to date, 251 patients were included. The authors reported a 5-year survival of 97% in patients without lymph node involvement, compared to a survival of 52% in those with lymph node involvement.40
Conclusions About Sentinel Lymph Node Biopsy in Merkel Cell CarcinomaBy way of conclusion, and according to the 2010 NCCN guidelines, when there is no clinical or radiologic evidence of lymph node involvement in most patients with Merkel cell carcinoma, sentinel lymph node biopsy should be performed as part of the routine management of the tumor (regardless of size), unless the procedure is contraindicated for other medical reasons.
One exception would be Merkel cell carcinoma located on the head and neck. In this case, the rate of false negatives is very high and so the technique is not routinely recommended, even though it may provide prognostic information.
Sentinel Lymph Node Biopsy in Other Skin TumorsSentinel Lymph Node Biopsy in Extramammary Paget DiseaseExtramammary Paget disease is a term for a type of rare tumor usually located on the genitals, although it has been reported at many other sites. Clinically, it presents as a hypo- or hyperpigmented erythematous plaque with poorly defined edges. The rate of local recurrence is high. Generally, prognosis is good, as the tumor cells tend to remain in the epidermis and rarely metastasize. Nevertheless, lymph node metastasis rates of up to 26% have been reported for invasive primary tumors.65
Management of patients with extramammary Paget disease, with no clinical evidence of lymph node involvement, is subject to debate. In patients with an invasive primary tumor with no clinical evidence of lymph node involvement, elective lymphadenectomy is recommended. Sentinel lymph node biopsy might be a useful approach in these patients.
In 2008, Hatta et al66,67 published a series of 76 patients, 24 of whom had undergone sentinel lymph node biopsy. The biopsy was positive in 12, and these patients underwent complete lymph node dissection. Among the parameters analyzed in this study was degree of tumor invasion. Tumors were thus divided into tumors that were wholly within the epidermis, those with microinvasion of the papillary dermis, and those with invasion of the reticular dermis and subcutaneous cell tissue. The authors found that 43 of the 76 patients studied (57%) had intraepidermal tumors, 22 (29%) had tumors with microinvasion of the papillary dermis, and 22 (14%) had tumors with invasion of the reticular dermis and subcutaneous cell tissue.
The authors’ univariate analysis found that patients with tumors showing invasion of the reticular dermis and subcutaneous cell tissue had a higher risk of mortality than those with intraepidermal tumors or those with tumors showing microinvasion of the papillary dermis (P=.01). They concluded that sentinel lymph node biopsy would be indicated in this study and in another conducted in 13 patients, all with invasive extramammary Paget disease, as the frequency of lymph node involvement, even when not clinically evident, is greater than in noninvasive disease.
The technique normally used for detection in sentinel lymph node biopsy is less sensitive for detecting lymph nodes located close to the tumor in extramammary Paget disease, as the radiotracer signal for the node overlaps with that of the primary tumor. In these cases, some authors have suggested that fluorescence with indocyanine green, detected with an infrared camera, could be useful.6
Sentinel Lymph Node Biopsy in Sweat Gland CarcinomaAdenocarcinomas of sweat glands form a heterogeneous group of tumors derived from eccrine or apocrine sweat glands. Given the low incidence of these tumors, randomized controlled trials are difficult to perform. Several studies have reported that these tumors, and aggressive digital papillary adenocarcinoma and the hidradenocarcinomas in particular, often spread to regional lymph nodes. In general, adnexal adenocarcinomas do not share a typical clinical presentation. The tumor grows rapidly, and bleeding and ulceration are common. Biopsy is usually required for diagnosis.
Little has been reported in the literature about sentinel lymph node biopsy in these patients.68–70 The largest study in which the procedure was used included 6 patients with sweat gland adenocarcinomas (3 hidradenocarcinomas, 2 eccrine duct cell carcinomas, and a porocarcinoma).71 Sentinel lymph node biopsy was positive in 4 patients, 2 of whom underwent regional lymphadenectomy while the other 2 did not undergo the procedure for medical reasons. All patients were alive and free of disease at the end of follow-up, which ranged from 2 to 19 months, with a mean of 12 months.
The authors indicated that although sentinel lymph node biopsy could be useful for staging in these patients, the prognostic value or usefulness for guiding treatment decisions would need studies with larger numbers of patients.
Sentinel Lymph Node Biopsy in Sebaceous Gland CarcinomaSebaceous gland carcinoma is an uncommon tumor that arises in the adnexal epithelium of sebaceous glands. It is most frequently located in the palpebral region (75%), although nonocular sites have also been reported. Clinical diagnosis is difficult and the tumor can easily be mistaken for other skin tumors or chronic inflammatory disease. The most frequent ocular presentation is in the form of a solitary, small, firm, raised nodule of yellow-orange color.
Nonocular tumors are usually on the head and neck, and their appearance is that of a yellow-orange nodule.
The tumor is aggressive and associated with high mortality and metastasis rates. Mortality rates range from 9% to 50%, but it seems that with early diagnosis these rates are declining.72
The mortality rates for ocular sebaceous carcinoma range from 11% to 30%, with distant metastases in 3% to 25%. In principle, it was thought that nonocular sebaceous carcinoma had a better prognosis than its ocular counterpart, but in a study of 91 patients with nonocular sebaceous carcinoma, a recurrence rate of 29% was reported with a metastasis rate of 21%.73
Several articles report studies of sentinel lymph node biopsy in ocular sebaceous carcinomas, suggesting that the technique is safe and simple, and that it could be useful for better staging and clinical management.
When the authors performed sentinel lymph node biopsy on 10 patients with sebaceous carcinoma on the eyelid (primary tumor in 4 and recurrent tumor in 6), the sentinal node was negative in all, but lymph node metastasis was detected in 2 of the 10 patients during follow-up. Despite these findings, the authors concluded that sentinel lymph node biopsy is a safe technique and that it should be considered in sebaceous carcinomas with a high risk of metastasis (recurrent, large size, or those involving postseptal structures).74,75
Nijhawan et al76 studied 6 patients with sebaceous carcinoma of the eyelid with clinically negative lymph nodes, and 5 of them underwent sentinel lymph node biopsy. This was negative in all cases, although the authors concluded that the technique could be useful given the high rate of lymph node involvement reported for such patients.
By way of conclusion, we could say that the data currently available do not support the usefulness of this technique in sebaceous carcinoma.
Sentinel Lymph Node Biopsy in Cutaneous LymphomasSentinel lymph node biopsy could play an important part in staging of primary cutaneous lymphomas. Several authors point out that, in their experience, lymph node involvement is the first stage of extracutaneous spread.
Very little has been published on the topic, however, and so larger, robustly designed studies would be needed to substantiate the theories put forward by these authors.63,77–79 Our literature review did not find any study that concluded that sentinel lymph node biopsy can determine whether lymph node involvement is primary or an extension of a cutaneous lymphoma, only whether there is lymph node involvement or not.
ConclusionsWith the data currently available, sentinel lymph node biopsy can be recommended for certain aggressive tumors, such as squamous cell carcinoma of the skin more than 6mm thick, Merkel cell carcinoma, invasive extramammary Paget disease, and perhaps sweat gland carcinomas.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Salguero-Fernández I, et al. Ganglio centinela en cáncer de piel no melanoma. Actas Dermosifiliogr.2011;102:589-598.