Laboratory diagnosis of the dermatomycoses is based on identification of the causative species by culture and by direct examination.1,2 The aim of this latter technique is to detect the fungi directly in the pathology sample using simple reagents or stains.
Direct examination has several advantages: it aids diagnosis in clinically atypical presentations (as occurs with tinea incognito); it provides diagnostic security, which is essential when costly, long-term treatments with potential side effects are to be initiated, as may be required for tinea capitis and the onychomycoses; it can suggest the probable causative fungi (for example, from the pattern of parasitization of the hair in cases of tinea capitis); and it favors therapeutic compliance by the patient.1,2 Despite this, the majority of Spanish dermatologists do not routinely perform direct examination. The video accompanying this article presents a review of this procedure.
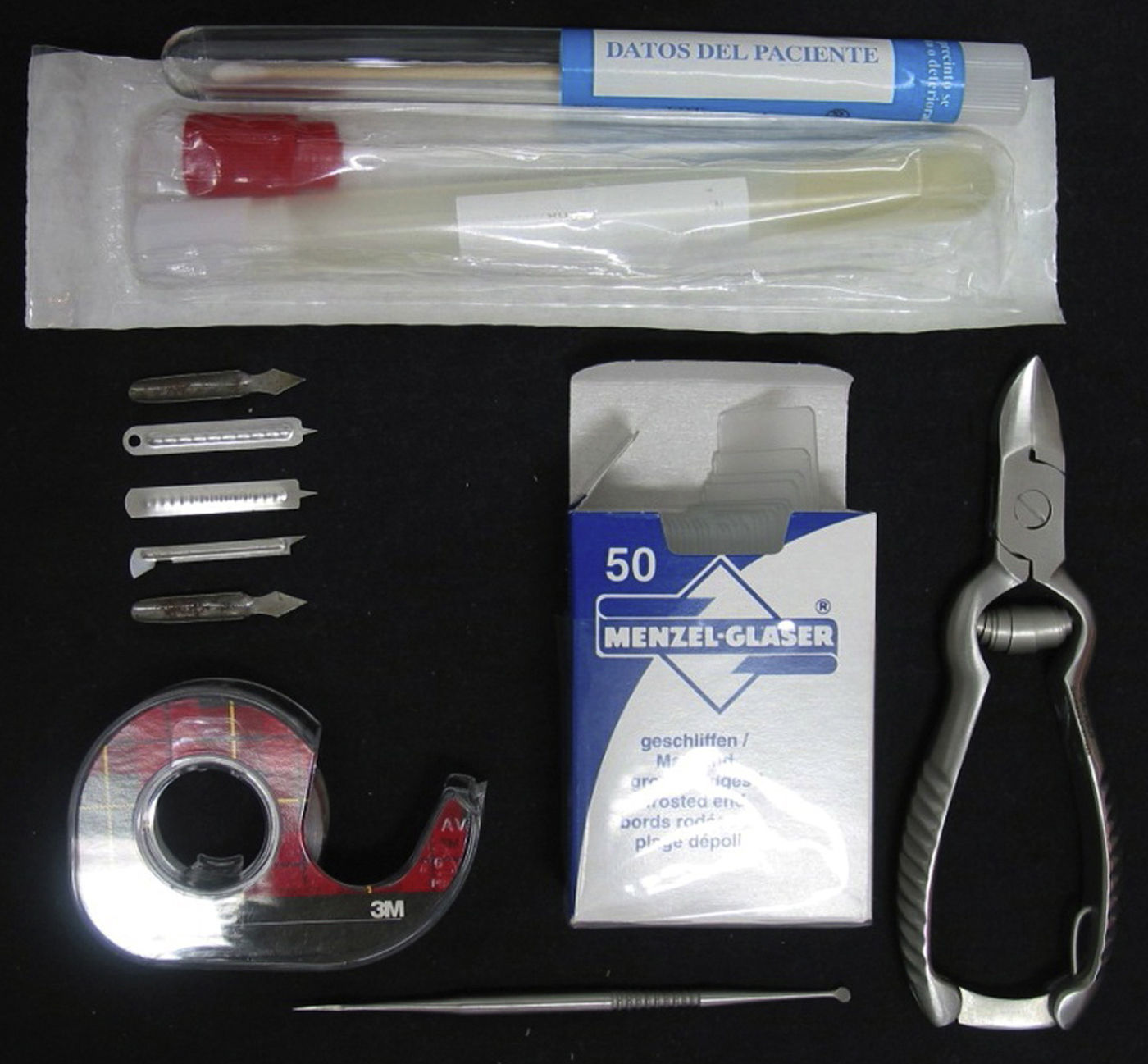
Description of the TechniqueFirst, a correct sampling technique is essential. Antifungal treatments must be interrupted at least 2 weeks before obtaining the sample. Instruments appropriate for the type of sample should be used (Fig. 1).1–3 In moist lesions it is better to use a brush or swab, whereas in dry lesions it is better to use a scalpel blade or lancet. In the case of samples from a nail, manicure clippers (or else curved scissors), and the Le Cron knife, commonly used in dentistry, are all practical alternatives.1
It can also be useful to take samples from skin lesions using sterile carpet, which can then be placed on the culture medium, making detection of bacterial infection or superinfection possible. This is important in tinea pedis, which not infrequently coexists with intertrigo due to Pseudomonas or other gram-negative bacteria or due to Candida infection. However, taking samples using sterile carpet makes it difficult to perform direct examination.
Prior to sampling, the area must be cleansed gently with a swab or cotton-wool soaked in 70o alcohol. The sample must be taken from the border of the lesion (the most active area). In tinea capitis, we must also aim to include some hair follicles in the sample (fragments of affected hairs are often found; on rubbing the area, these hairs typically become loose and pulling is not required). When distal onychomycosis is suspected, the most distal area of onycholysis should be removed and the sample taken more proximally.1
An effort should be made to obtain sufficient material. Part of the material obtained is deposited in the center of a microscope slide (or, if the sample is obtained by brushing, the brush is wiped against the slide) and a drop of the chosen stain is placed on the surface. At the present time, the most widely used is Swartz-Lamkins solution (Parker permanent black ink in equal parts with 20% potassium hydroxide), though other stains, such as those that combine potassium hydroxide and calcofluor, are also employed.1,4 The preparation is then covered by a coverslip.1
When the material contains scales, the keratinous material can be dissolved by gently heating the preparation over a cigarette lighter flame or by pressing gently and repeatedly on the coverslip. The sample is then examined by microscopy, first at low magnification (×10) and then at high magnification (×20 and ×40).
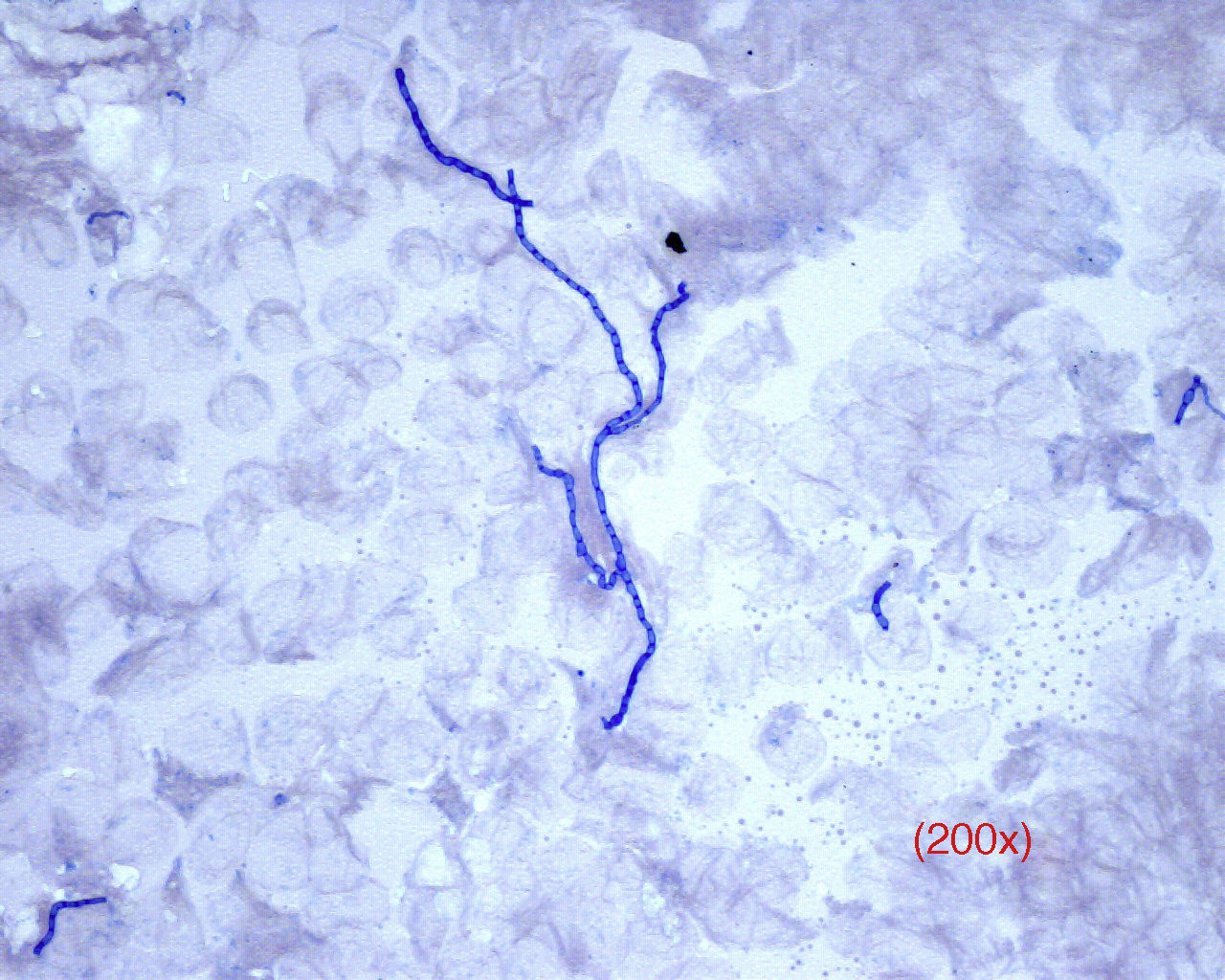
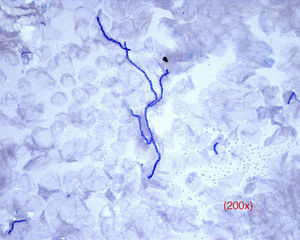
On direct examination, dermatophytes appear as septate and branching filaments with clear, regular hyaline borders that slowly take up the blue color from the ink (Fig. 2). In cases of tinea capitis, special attention should be paid to parasitization of the hair (either externally, ectothrix, or internally, endothrix) to determine the best antifungal therapy while waiting for the culture results (griseofulvin for ectothrix and terbinafine for endothrix).
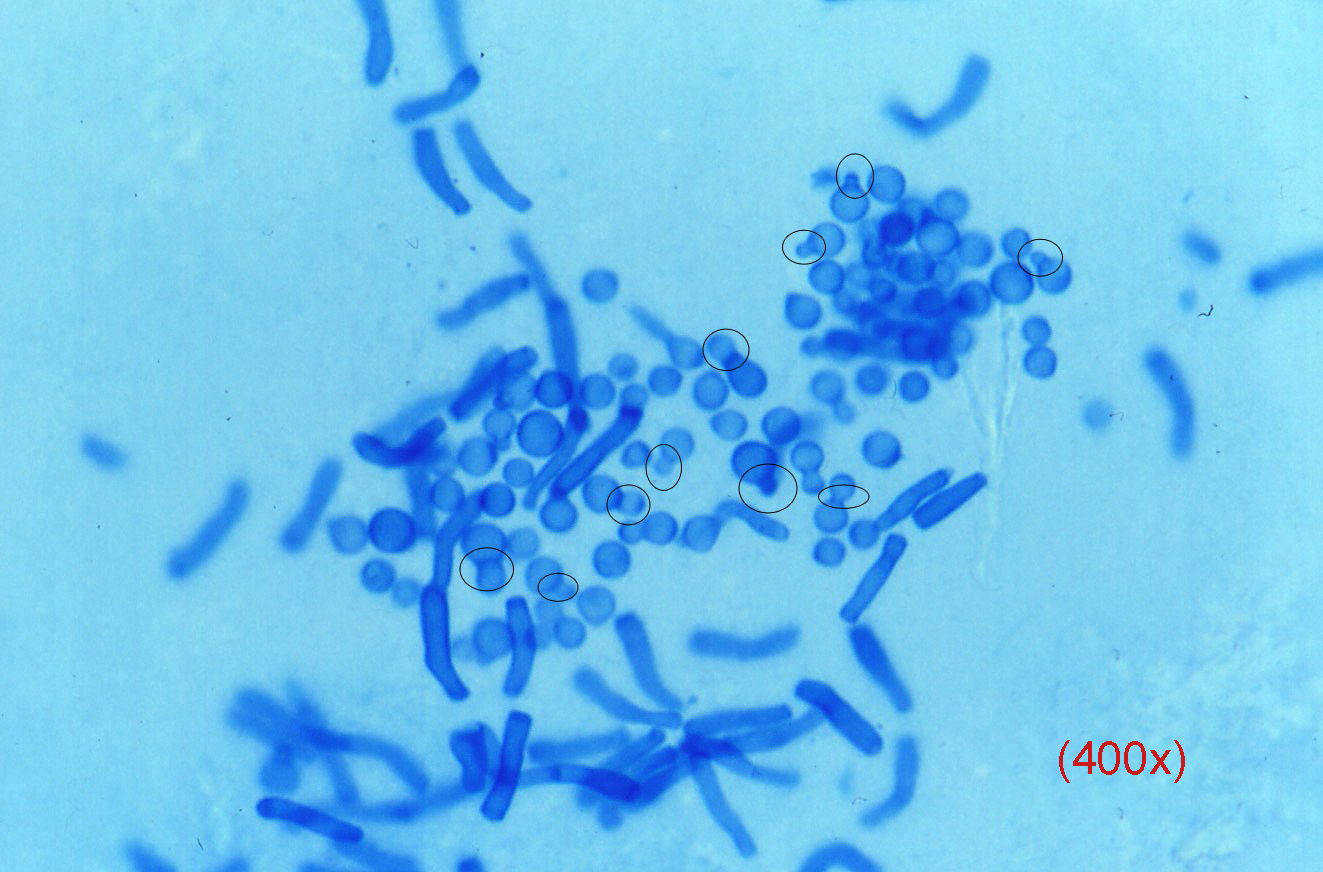
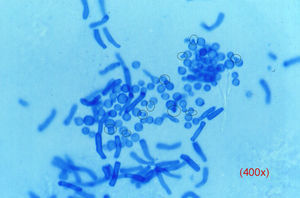
Yeasts, which can be difficult to detect, usually present as budding blastoconidia and pseudohyphae. Pityriasis versicolor produces a pathognomic image (Fig. 3) in which there is a mixture of individual blastospores with clear collarettes and short and thick pseudohyphae; these structures stain very rapidly.
Opportunistic moulds rarely show up on direct examination, except in the onychomycoses, in which hyphae and conidia can often be observed; these have a specific morphology that differs from dermatophytes and yeasts.
Advantages and Disadvantages of Direct ExaminationThe advantages of this technique include the large amount of information rapidly provided, at low cost and with minimal instrument requirements. Furthermore, it has a high sensitivity, it enables us to distinguish between dermatophytes and Candidas, and it is particularly useful in atypical cases (tinea incognito) and in the onychomycoses, in which its sensitivity is much higher than that of culture.1,4,5 A positive direct examination is considered a necessary criterion to establish pathogenicity in suspected onychomycosis due to non-albicans Candida or nondermatophyte moulds.
A disadvantage, as with any technique, is the learning curve required. In addition, it is time consuming and does not distinguish between species.1
ConclusionsA correct technique when taking samples and the performance of direct examination is very important in the investigation of a possible dermatomycosis, although mycology study must be completed by performing culture.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We would like to thank the Dermatology Departments of Hospital Costa del Sol in Marbella and Hospital Carlos Haya in Malaga, for their help in preparing this publication.
Please cite this article as: del Boz J, Padilla-España L, Crespo-Erchiga V. Toma de muestras y examen directo en dermatomicosis. Actas Dermosifiliogr. 2016;107:64–66.