Pemphigus is an autoimmune disease that causes blisters and erosions and is characterized by autoantibodies to desmogleins. Its prognosis was dismal until 1959, when the introduction of corticosteroids revolutionized treatment and drastically reduced associated deaths.1 Corticosteroids have been the first-line treatment for pemphigus ever since. Long-term high-dose corticosteroid therapy achieves high remission rates, but comes with considerable adverse effects. Immunosuppressive drugs and immunomodulators are often used as corticosteroid-sparing agents in an attempt to reduce these effects, but there is limited evidence on their effectiveness (Table 1).2
Key Points in the Treatment of Pemphigus.
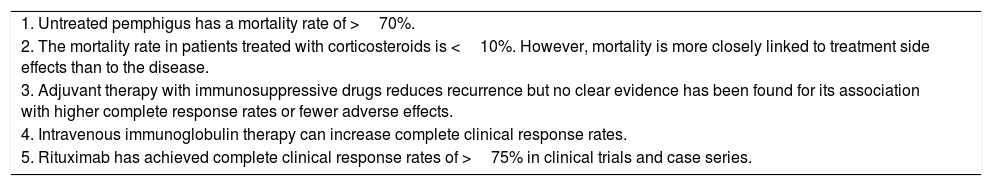
| 1. Untreated pemphigus has a mortality rate of >70%. |
| 2. The mortality rate in patients treated with corticosteroids is <10%. However, mortality is more closely linked to treatment side effects than to the disease. |
| 3. Adjuvant therapy with immunosuppressive drugs reduces recurrence but no clear evidence has been found for its association with higher complete response rates or fewer adverse effects. |
| 4. Intravenous immunoglobulin therapy can increase complete clinical response rates. |
| 5. Rituximab has achieved complete clinical response rates of >75% in clinical trials and case series. |
Rituximab (RTX) is an anti-CD20 monoclonal antibody that has been used off-label to treat pemphigus for several decades. It is used particularly to treat patients with refractory disease or who are unable to tolerate conventional treatment.3 The results of the first multicenter, prospective, open-label, randomized trial comparing the efficacy of RTX combined with low-dose prednisone (RTX-LDPRD) with that of long-term high-dose prednisone as monotherapy (HD-PDN) were recently published.4 The study included 90 adults recently diagnosed with untreated pemphigus vulgaris or pemphigus foliaceus. The RTX-LDPRD group received 1g of RTX on days 0 and 14, followed by 500mg at months 12 and 18 together with 0.5 or 1mg/kg/d of prednisone tapered over 3 months for moderate disease or 6 months for severe disease. The HD-PRD group received 1 or 1.5mg/kg/d of prednisone tapered over 12 or 18 months (for moderate and severe disease, respectively). The follow-up period for both groups was 36 months. Significant results were observed at 24 months: 89% (41/46) of the patients in the RTX-LDPRD group achieved complete remission without treatment (CR-WT) versus 34% (15/44) of those in the HD-PRD group (relative risk, 2.61; 95% CI, 1.71-3.99; p<.0001). Time to CR-WT was shorter in the RTX-LDPRD group (277 vs. 677 days) and these patients also experienced longer disease-free periods (466 vs. 62 days, representing a 7-fold difference). The cumulative dose of corticosteroids was 3 times lower in patients treated with RTX-LDPRD, who also had fewer serious adverse effects (27 events in 16 patients vs. 53 events in 29 patients). There were no differences in number of infections. The most common adverse effects were endocrine, muscular, and bone disorders. The recurrence rate in the first 24 months was 24% in the RTX-LDPRD group and 45% in the HD-PRD group. Just 1 of the patients in the RTX-LDPRD group who achieved CR-WT at month 24 experienced recurrence over the next 12 months. In brief, patients treated with RTX combined with low-dose corticosteroids responded better, faster, and for longer and also had fewer recurrences and adverse effects. Treatment cost per patient was 5500 Euro more expensive in the RTX-LDPRD group but this difference is of little significance if we consider the associated reductions in adverse effects, work absenteeism, and indirect health costs. The results of this trial suggest that RTX could revolutionize the treatment of pemphigus, replacing corticosteroid therapy as the first-line treatment option.
Please cite this article as: Morgado-Carrasco D, Giavedoni P, Fustà-Novell X, Iranzo P. FR-Rituximab, una revolución en el tratamiento del pénfigo. Actas Dermosifiliogr. 2018;109:177–178.