While locoregional cutaneous metastases (in transit and satellite) in melanoma have received little attention from researchers to date, they have pathogenic and prognostic features that distinguish them from other forms of locoregional recurrence. Identifying predictors of these metastases would be of great value for their prevention, early diagnosis, and treatment. The aim of this study was to identify the risk factors associated with locoregional cutaneous metastases as the first form of recurrence in the metastatic progression of melanoma.
Material and methodsBetween 2000 and 2010, we prospectively collected the data of 1327 patients diagnosed with stage I and II melanoma. During follow up, 112 patients (8.4%) developed metastases. Of these, 36 had exclusively locoregional cutaneous metastases. The clinical and histological characteristics of this subgroup were evaluated.
ResultsIn the univariate analysis, significant predictors were patient age, primary tumor thickness, site, ulceration, mitotic index, and histological type. After multivariate analysis, the independent risk factors were tumor thickness (risk ratio [RR] 5.6; 95% CI: 2.7-11.5) and the location of the primary tumor on the lower limbs (RR 3.4; 95% CI: 1.0-11.5), on the head or neck (RR 4.8; 95% IC: 1.7-13.5), or in acral sites (RR 6.7; 95% IC: 2.2-20.8).
ConclusionPatients who have melanomas with a Breslow thickness of more than 2mm located on the lower limbs, head, neck, or acral sites have a higher risk of developing locoregional cutaneous metastases. These findings could be useful in the design of future guidelines for the monitoring and management of melanoma.
Las metástasis cutáneas locorregionales (en tránsito y satelitosis) constituyen un evento poco estudiado en la progresión del melanoma, con diferencias patogénicas y pronósticas respecto a otras formas de recaída locorregional. Conocer las variables predictivas de este evento sería de gran utilidad en su prevención, diagnóstico precoz y tratamiento. El objetivo de este trabajo fue evaluar los posibles factores de riesgo asociados a la aparición de metástasis cutáneas locorregionales como primera forma de recaída en la progresión metastásica del melanoma.
Material y métodosEntre 2000 y 2010, los datos de 1.327 pacientes diagnosticados de melanoma en estadios i y ii fueron recogidos de forma prospectiva en nuestras consultas. Durante el seguimiento, un total de 112 (8,4%) pacientes sufrió progresión metastásica de su enfermedad. De ellos, 36 pacientes presentaron metástasis cutáneas locorregionales no concurrentes con otras formas de recurrencia. Las características clínicas e histológicas de este subgrupo fueron evaluadas.
ResultadosEn el análisis univariante, los factores predictivos significativos fueron la edad del paciente, el espesor del tumor primario, la localización, la ulceración, el índice mitósico y el tipo histológico. Después del análisis multivariante, se mantuvieron como factores de riesgo independientes el espesor (razón de riesgo [RR] 5,6 e IC 95%: 2,7-11,5), la localización del tumor primario en miembros inferiores (RR 3,4 e IC 95%: 1,0-11,5), en cabeza/cuello (RR 4,8 e IC 95%: 1,7-13,5) y en zonas acrales (RR 6,7 e IC 95%: 2,2-20,8).
ConclusiónLos pacientes con melanomas de más de 2mm de Breslow, localizados en miembros inferiores, cabeza/cuello y zonas acrales tienen un mayor riesgo de padecer metástasis cutáneas locorregionales. Estos datos podrían ser útiles en el diseño de futuras guías para el seguimiento y manejo del melanoma.
Cutaneous melanoma, a tumor with high metastatic potential, is considered to be among the cancers with the fastest growing incidence in white populations.1
Twenty percent of patients who develop recurrent cutaneous melanoma have exclusively locoregional cutaneous involvement (local recurrence, satellite metastases, or in-transit metastases).2,3 In locally recurrent melanoma (in which tumor cells are present in the primary tumor excision scar), a distinction is made between recurrences that are assumed to be satellite metastases and recurrences that might be caused by the persistence of tumor cells from an incompletely excised primary tumor. Satellite metastases and/or in-transit metastases usually precede lymph node metastases, whereas the development of distant metastases appears to be an independent event in neoplastic spread.2,4 Few studies have evaluated the risk factors associated with exclusively locoregional cutaneous tumor progression in patients with melanoma5–13 but an association has been reported between risk of recurrence and the clinical and pathologic characteristics of the primary tumor (thickness, site, ulceration), extent of disease, and patient age and sex. Other factors, such as mitotic index, have been examined by very few studies.12,14 In the specific case of intralymphatic (in-transit and satellite) metastases, the location of the primary tumor on the lower limbs, intralymphatic invasion, and the presence of lymph node metastases have been identified as risk factors.5,6,15–20 These last 2 characteristics are used to classify stage III patients according to the most recent staging criteria published by the American Joint Committee on Cancer (AJCC).8 However, no previous studies have evaluated in patients without previous lymphatic involvement (locoregional cutaneous or lymph node metastases) the risk factors associated with in-transit and satellite cutaneous metastases as an isolated event in the progression of melanoma, in the absence of other forms of recurrence (lymph node or distant metastases). It is of fundamental importance to understand and characterize the specific risk factors associated with locoregional cutaneous metastasis because it differs from other forms of tumor progression in terms of prognosis and management.8
The primary objective of this study was to evaluate the possible clinical and pathologic risk factors for the development of locoregional cutaneous metastases as the first and sole form of metastatic spread in patients with cutaneous melanoma.
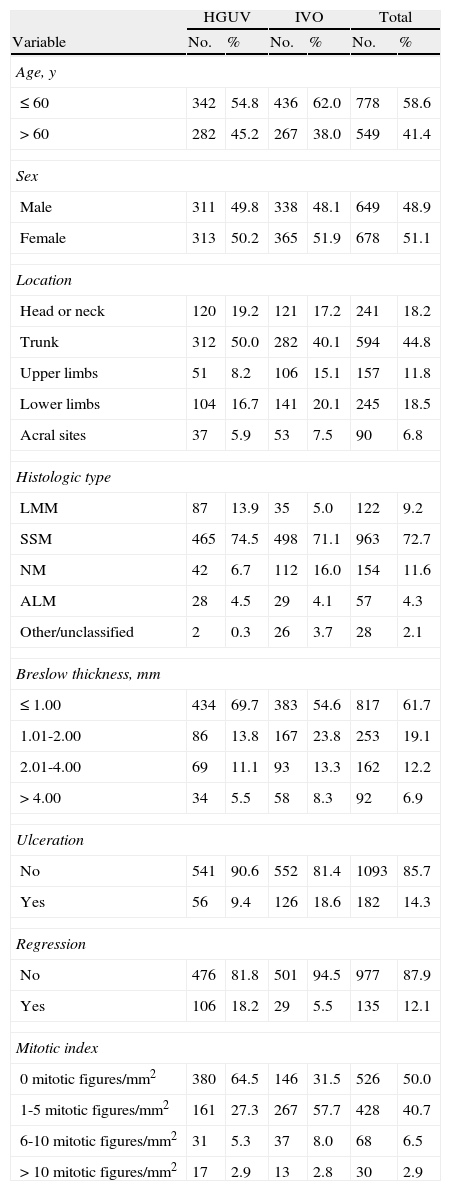
Material and MethodsA total of 1327 consecutive patients diagnosed with primary localized cutaneous melanoma (pathologic stages I and II)8 between January 2000 and July 2010 were identified in the melanoma databases of the Instituto Valenciano de Oncología and the Consorcio Hospital General Universitario de Valencia in Valencia, Spain.21Table 1 shows the characteristics of the patients included in the study. Patients with a primary tumor in an unknown or noncutaneous location or with a second primary melanoma, and, by definition, patients with microsatellites or vascular invasion (AJCC classification NIIc) and/or a positive sentinel node biopsy (SNB) were excluded from the study.
Characteristics of the 1327 Patients Included in the Study.
| HGUV | IVO | Total | ||||
| Variable | No. | % | No. | % | No. | % |
| Age, y | ||||||
| ≤60 | 342 | 54.8 | 436 | 62.0 | 778 | 58.6 |
| >60 | 282 | 45.2 | 267 | 38.0 | 549 | 41.4 |
| Sex | ||||||
| Male | 311 | 49.8 | 338 | 48.1 | 649 | 48.9 |
| Female | 313 | 50.2 | 365 | 51.9 | 678 | 51.1 |
| Location | ||||||
| Head or neck | 120 | 19.2 | 121 | 17.2 | 241 | 18.2 |
| Trunk | 312 | 50.0 | 282 | 40.1 | 594 | 44.8 |
| Upper limbs | 51 | 8.2 | 106 | 15.1 | 157 | 11.8 |
| Lower limbs | 104 | 16.7 | 141 | 20.1 | 245 | 18.5 |
| Acral sites | 37 | 5.9 | 53 | 7.5 | 90 | 6.8 |
| Histologic type | ||||||
| LMM | 87 | 13.9 | 35 | 5.0 | 122 | 9.2 |
| SSM | 465 | 74.5 | 498 | 71.1 | 963 | 72.7 |
| NM | 42 | 6.7 | 112 | 16.0 | 154 | 11.6 |
| ALM | 28 | 4.5 | 29 | 4.1 | 57 | 4.3 |
| Other/unclassified | 2 | 0.3 | 26 | 3.7 | 28 | 2.1 |
| Breslow thickness, mm | ||||||
| ≤1.00 | 434 | 69.7 | 383 | 54.6 | 817 | 61.7 |
| 1.01-2.00 | 86 | 13.8 | 167 | 23.8 | 253 | 19.1 |
| 2.01-4.00 | 69 | 11.1 | 93 | 13.3 | 162 | 12.2 |
| >4.00 | 34 | 5.5 | 58 | 8.3 | 92 | 6.9 |
| Ulceration | ||||||
| No | 541 | 90.6 | 552 | 81.4 | 1093 | 85.7 |
| Yes | 56 | 9.4 | 126 | 18.6 | 182 | 14.3 |
| Regression | ||||||
| No | 476 | 81.8 | 501 | 94.5 | 977 | 87.9 |
| Yes | 106 | 18.2 | 29 | 5.5 | 135 | 12.1 |
| Mitotic index | ||||||
| 0 mitotic figures/mm2 | 380 | 64.5 | 146 | 31.5 | 526 | 50.0 |
| 1-5 mitotic figures/mm2 | 161 | 27.3 | 267 | 57.7 | 428 | 40.7 |
| 6-10 mitotic figures/mm2 | 31 | 5.3 | 37 | 8.0 | 68 | 6.5 |
| >10 mitotic figures/mm2 | 17 | 2.9 | 13 | 2.8 | 30 | 2.9 |
Abbreviations: ALM, acral lentiginous melanoma; HGUV, Consorcio Hospital General Universitario de Valencia; IVO, Instituto Valenciano de Oncología; LMM, lentigo maligna melanoma; NM, nodular melanoma; SSM, superficial spreading melanoma.
The dependent variable in the statistical analysis was the presence of locoregional cutaneous metastases during follow-up as the first and sole form of recurrence of disease. Therefore, patients with concurrent lymph node involvement or systemic metastasis were also excluded.
For the purposes of the analysis, the locoregional cutaneous metastases were divided into 3 groups: local recurrences (in the tumor excision scar), satellite metastases (≤5cm from the original primary tumor), and in-transit metastases (>5cm from the primary tumor). Local recurrences with clinical or histologic features suggestive of primary tumor growth at the site of an incomplete excision according to criteria described in previous studies22,23 were also excluded from the analysis.
All patients were managed according to the guidelines for cutaneous melanoma published under the umbrella of the oncology plan of the Autonomous Community of Valencia.24 In accordance with these guidelines, following the diagnosis of melanoma, a wide excision was performed on the primary tumor or, in patients with a biopsy or excision scar, the margins were increased. The excision/widening margins were 1cm for melanomas with a Breslow thickness of less than2mm and 2cm for thicker tumors. In our units, SNB was performed on patients with a primary melanoma at least0.75mm thick or a thinner primary tumor with ulceration and/or regression.
In accordance with our protocol, patient follow-up included a clinical examination at each visit and laboratory and radiographic tests (chest radiograph, abdominal ultrasound, ultrasound of regional lymph nodes, and computed tomography scan of the chest and abdomen). Patients attended follow-up visits every 3 to 6 months for the first 3 years, every 6 to 12 months for the following 2 years, and once a year thereafter. All patients underwent radiographic examination at the time of diagnosis and every 6 to 12 months for the first 5 years of follow-up.
The aim of this study was to identify the possible risk factors associated with the development of locoregional cutaneous metastases as the sole form of recurrence. Both clinical and histologic variables were selected for analysis. The clinical variables analyzed were age (≤60 or >60 years), sex (male or female), and primary tumor location (head or neck, trunk, lower limbs, or upper limbs).
The histopathologic variables analyzed were histologic type (lentigo maligna melanoma, superficial spreading melanoma, nodular melanoma, acral lentiginous melanoma, and other/unclassified), Breslow thickness (≤2 or >2mm), presence or absence of ulceration, presence or absence of regression, and mitotic index of the primary tumor. Mitotic index was measured in mitotic figures per mm,2 evaluated in 1mm2 in the area of highest mitotic activity and classified as either <6 or ≥6 mitotic figures per mm2; we chose the cutoff point of 6 mitotic figures because it has been found to best predict risk of recurrence.25
The differences in risk of locoregional cutaneous metastases associated with the study variables were calculated using the Kaplan-Meier method and evaluated using the log-rank test. Multivariate analysis of the predictors of locoregional cutaneous metastases was conducted using the Cox proportional hazard model. For all tests, statistical significance was set at a P value of less than.05.
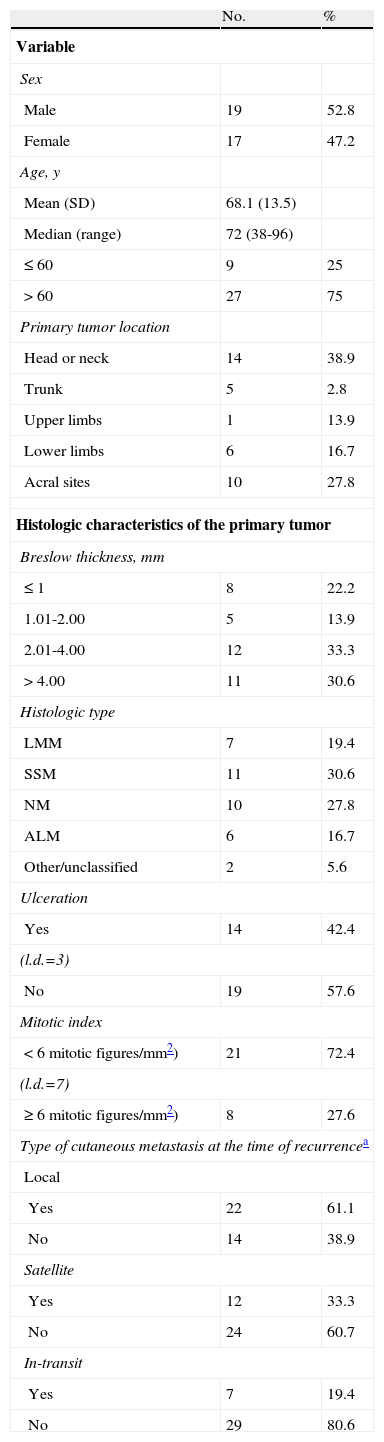
ResultsDuring a median follow-up period of 37 months (interquartile range, 13-62 months), tumor progression was observed in 112 (8.4%) of the 1327 patients with AJCC stage I or II melanoma. A subgroup of 36 patients (32% of those with recurrent disease) had locoregional cutaneous metastases as the first and sole form of progression—in other words, in the absence of nodal recurrence or distant metastasis. Table 2 shows the characteristics of these 36 patients. The median time from excision of the primary tumor to the development of locoregional cutaneous metastases was 13.4 months (interquartile range, 6.4-23.6 months). Twenty-seven of the 36 patients had a single cutaneous metastasis at the time of diagnosis (18 local, 7 satellite, and 2 in-transit). Of the remaining 9 patients with 2 or more lesions at the time of recurrence, 1 had local lesions only, 2 had satellite metastases only, 2 had in-transit metastases only, and 4 had more than 1 form of locoregional cutaneous metastasis.
Characteristics of Patients with Locoregional Cutaneous Metastases as the First Form of Recurrence (n=36).
| No. | % | |
| Variable | ||
| Sex | ||
| Male | 19 | 52.8 |
| Female | 17 | 47.2 |
| Age, y | ||
| Mean (SD) | 68.1 (13.5) | |
| Median (range) | 72 (38-96) | |
| ≤60 | 9 | 25 |
| >60 | 27 | 75 |
| Primary tumor location | ||
| Head or neck | 14 | 38.9 |
| Trunk | 5 | 2.8 |
| Upper limbs | 1 | 13.9 |
| Lower limbs | 6 | 16.7 |
| Acral sites | 10 | 27.8 |
| Histologic characteristics of the primary tumor | ||
| Breslow thickness, mm | ||
| ≤1 | 8 | 22.2 |
| 1.01-2.00 | 5 | 13.9 |
| 2.01-4.00 | 12 | 33.3 |
| >4.00 | 11 | 30.6 |
| Histologic type | ||
| LMM | 7 | 19.4 |
| SSM | 11 | 30.6 |
| NM | 10 | 27.8 |
| ALM | 6 | 16.7 |
| Other/unclassified | 2 | 5.6 |
| Ulceration | ||
| Yes | 14 | 42.4 |
| (l.d.=3) | ||
| No | 19 | 57.6 |
| Mitotic index | ||
| <6mitotic figures/mm2) | 21 | 72.4 |
| (l.d.=7) | ||
| ≥6mitotic figures/mm2) | 8 | 27.6 |
| Type of cutaneous metastasis at the time of recurrencea | ||
| Local | ||
| Yes | 22 | 61.1 |
| No | 14 | 38.9 |
| Satellite | ||
| Yes | 12 | 33.3 |
| No | 24 | 60.7 |
| In-transit | ||
| Yes | 7 | 19.4 |
| No | 29 | 80.6 |
Abbreviations: ALM, acral lentiginous melanoma; l.d., lost data; LMM, lentigo maligna melanoma; NM, nodular melanoma; SD, standard deviation; SSM, superficial spreading melanoma.
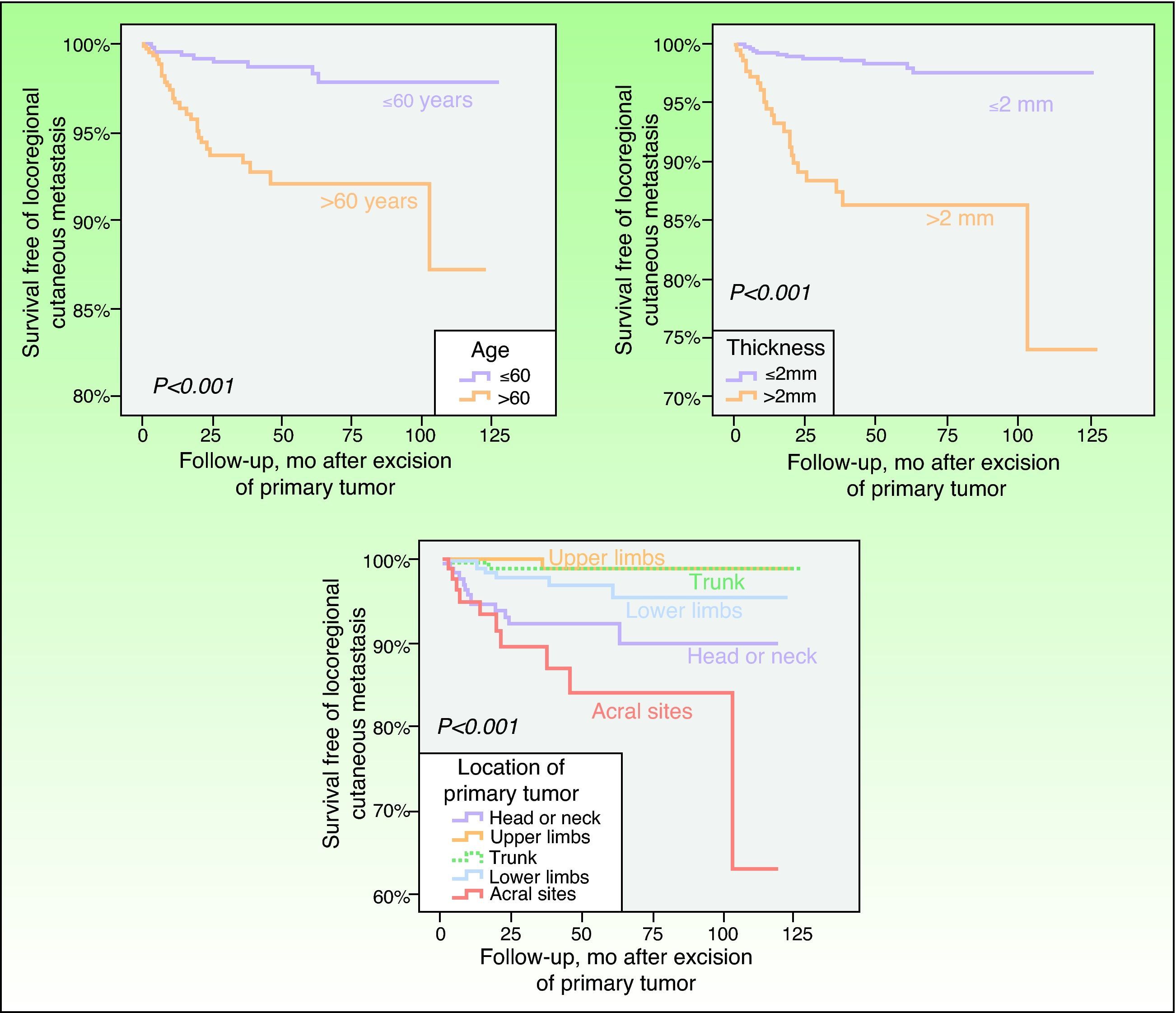
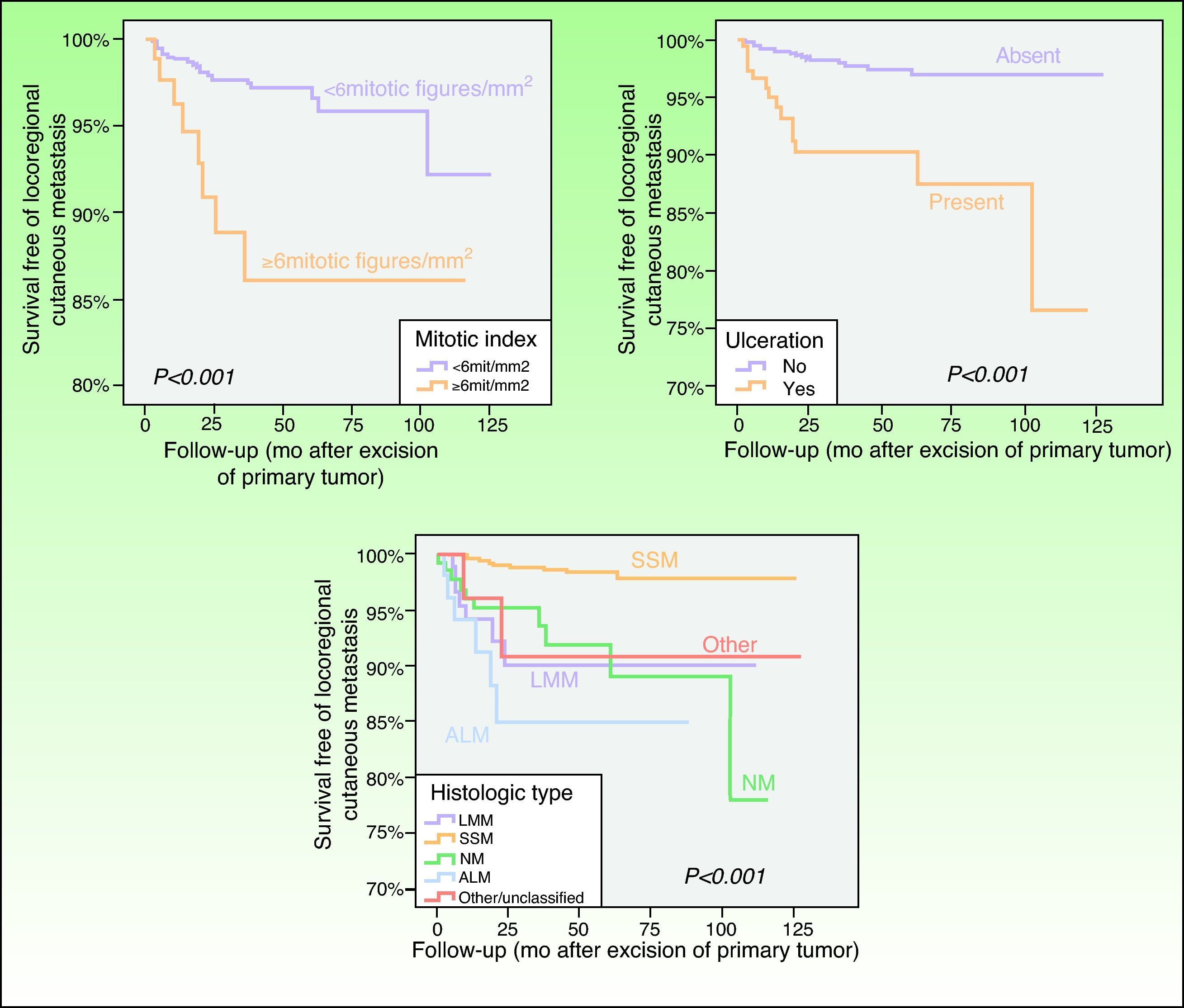
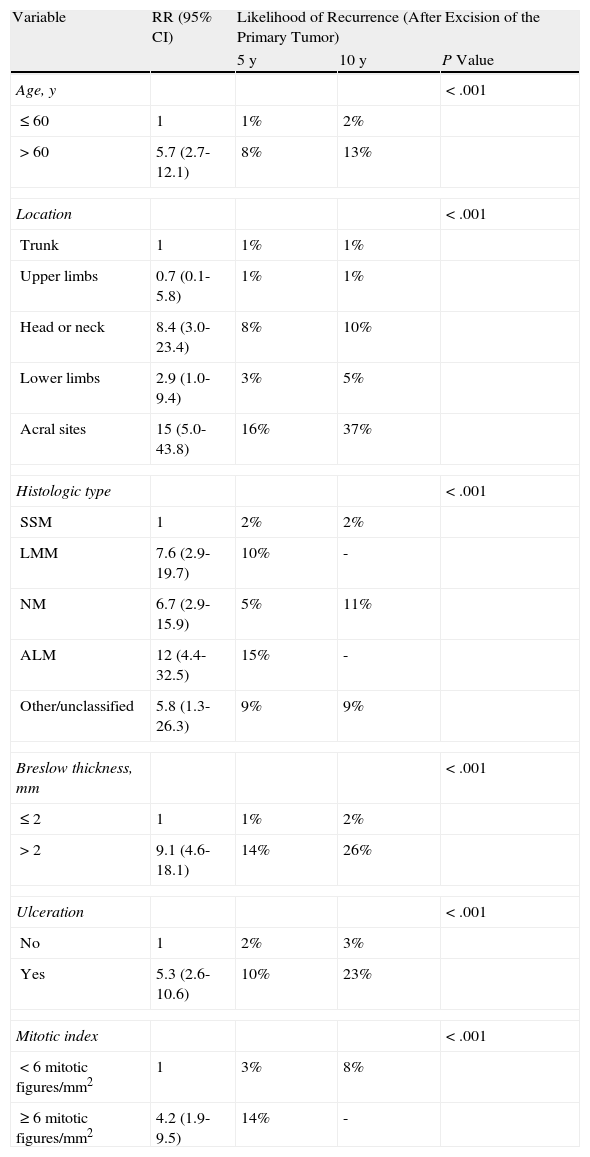
In the univariate analysis, the factors that had the most statistically significant influence on the likelihood of developing locoregional cutaneous metastases were primary tumor thickness, histologic type, ulceration, mitotic index, primary tumor location, and patient age (Table 3; Figs. 1 and 2). No differences in the risk of locoregional cutaneous metastases were found in relation to the patient's sex or to the presence or absence of primary tumor regression.
Univariate Analysis of the Factors Associated With Locoregional Cutaneous Metastases as the First Form of Recurrence.
| Variable | RR (95% CI) | Likelihood of Recurrence (After Excision of the Primary Tumor) | ||
| 5 y | 10 y | P Value | ||
| Age, y | <.001 | |||
| ≤60 | 1 | 1% | 2% | |
| >60 | 5.7 (2.7-12.1) | 8% | 13% | |
| Location | <.001 | |||
| Trunk | 1 | 1% | 1% | |
| Upper limbs | 0.7 (0.1-5.8) | 1% | 1% | |
| Head or neck | 8.4 (3.0-23.4) | 8% | 10% | |
| Lower limbs | 2.9 (1.0-9.4) | 3% | 5% | |
| Acral sites | 15 (5.0-43.8) | 16% | 37% | |
| Histologic type | <.001 | |||
| SSM | 1 | 2% | 2% | |
| LMM | 7.6 (2.9-19.7) | 10% | - | |
| NM | 6.7 (2.9-15.9) | 5% | 11% | |
| ALM | 12 (4.4-32.5) | 15% | - | |
| Other/unclassified | 5.8 (1.3-26.3) | 9% | 9% | |
| Breslow thickness, mm | <.001 | |||
| ≤2 | 1 | 1% | 2% | |
| >2 | 9.1 (4.6-18.1) | 14% | 26% | |
| Ulceration | <.001 | |||
| No | 1 | 2% | 3% | |
| Yes | 5.3 (2.6-10.6) | 10% | 23% | |
| Mitotic index | <.001 | |||
| <6 mitotic figures/mm2 | 1 | 3% | 8% | |
| ≥6 mitotic figures/mm2 | 4.2 (1.9-9.5) | 14% | - | |
Abbreviations: LMM, lentigo maligna melanoma; SSM, superficial spreading melanoma; ALM, acral lentiginous melanoma; NM, nodular melanoma; RR, risk ratio.
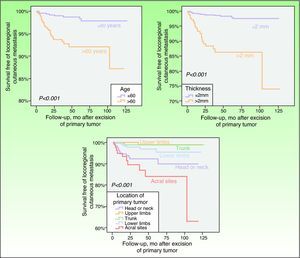
Kaplan-Meier curves showing that patients aged>60 years, with a Breslow thickness >2mm, or with primary tumors on the head or neck, on the lower limbs, or in acral sites, had a statistically significantly greater risk of developing locoregional cutaneous metastasis as the first and sole form of melanoma recurrence.
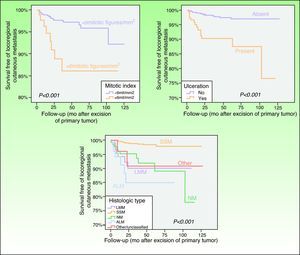
Disease-free survival curves showing significant differences in the likelihood of developing locoregional cutaneous metastasis associated with the mitotic index, ulceration, and the histologic type of the primary tumor. LMM indicates lentigo maligna melanoma; SSM, superficial spreading melanoma; NM, nodular melanoma; ALM, acral lentiginous melanoma.
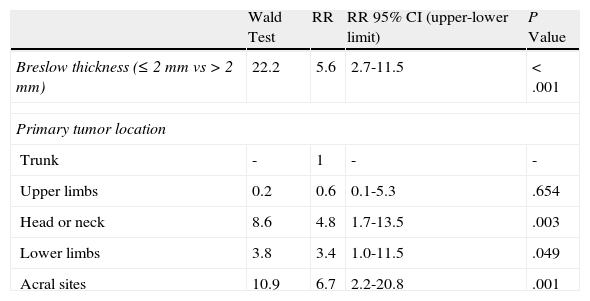
In the multivariate analysis, the factors that were statistically significant in the univariate analysis were introduced in the Cox proportional hazard model using forward stepwise selection of significant variables with adjustment for age and sex. The only variables that remained significant were Breslow thickness and primary tumor location (Table 4).
Multivariate Analysis of Risk Factors for Locoregional Cutaneous Metastases, Adjusted for Age and Sex.
| Wald Test | RR | RR 95% CI (upper-lower limit) | P Value | |
| Breslow thickness (≤2mm vs>2mm) | 22.2 | 5.6 | 2.7-11.5 | <.001 |
| Primary tumor location | ||||
| Trunk | - | 1 | - | - |
| Upper limbs | 0.2 | 0.6 | 0.1-5.3 | .654 |
| Head or neck | 8.6 | 4.8 | 1.7-13.5 | .003 |
| Lower limbs | 3.8 | 3.4 | 1.0-11.5 | .049 |
| Acral sites | 10.9 | 6.7 | 2.2-20.8 | .001 |
Abbreviations: RR, risk ratio.
This study evaluated the risk factors associated with the development of locoregional cutaneous metastases in a large series of patients. The large series was necessary because locoregional cutaneous metastases rarely occur without other forms of progession.8,13 The study used data from the databases of the dermatology department of the Instituto Valenciano de Oncología and the Consorcio Hospital General Universitario de Valencia because these 2 databases have a similar design and contain more than 3500 documented melanoma cases.21 For this study, we selected 1327 patients with stage I or II melanoma; of these, just 36 patients with locoregional cutaneous metastases (2.8%) were identified for subsequent analysis. This percentage is lower than those usually reported in the literature (>10%).2,3,26,27 However, it is easily explained by the relatively recent inclusion of SNB in the pathologic classification of melanoma and seems to be corroborated by data from more recent studies on the incidence of locoregional cutaneous metastases in patients without sentinel lymph node metastasis.5,20
Our study has several limitations, including its retrospective design, the short follow-up period, and the small number of patients with locoregional cutaneous metastases despite the considerable number of patients with melanoma analyzed. This last limitation is probably due to the fact that our follow-up period was shorter than that of other studies. Because of the small number of patients with locoregional cutaneous metastases included in the study, our findings should be interpreted with caution.The development of in-transit and/or satellite cutaneous metastasis is an early event in the lymphatic spread of melanoma that differs from other forms of locoregional recurrence in its pathogenesis and prognosis.5,6,8,28,29 Histopathology shows that locoregional cutaneous metastases develop as a result of tumor cell emboli entrapped in the dermal lymph vessels.27 Furthermore, several studies have found that visceral metastases in patients with locoregional cutaneous metastases are usually preceded by nodal recurrence,2,4,6 supporting the hypothesis that in-transit and satellite metastases have an intralymphatic origin. Intralymphatic metastasis is included in stage IIIB in the AJCC staging system because this stage is the closest statistical fit for this group in terms of prognosis. Nevertheless, patients with stage IIIB melanoma are a heterogeneous group, and those who present locoregional cutaneous metastases appear to have higher survival rates.3,8,15 The better prognosis seen in patients with locoregional cutaneous metastases could be due to the less aggressive biological behavior of this form of recurrence, although it could also be explained by the fact that locoregional cutaneous metastases respond better to the available treatments (excision, radiation therapy, isolated limb perfusion, and topical imiquimod).20,30–32
Melanoma recurrences are usually characterized by unsatisfactory management and a grim prognosis. The ability to identify at-risk patients would therefore be very useful, as it would allow preventive measures to be applied or, at the very least, make early diagnosis and treatment possible. The ability to predict potentially treatable forms of progression such as locoregional cutaneous metastasis would be of particular interest.
In our study, the only significant independent risk factors for locoregional cutaneous metastasis as the first and sole form of metastatic spread were the thickness and location of the primary tumor. We therefore consider that patients at the greatest risk of developing locoregional cutaneous metastases (those who have a tumor >2mm thick on the head or neck, on the lower limbs, or in acral sites) could benefit from closer clinical monitoring, as this would facilitate early diagnosis.
Several studies have found locoregional cutaneous metastases to be associated with primary tumor thickness and lymphatic involvement. Greater thickness may increase the likelihood that a tumor will come into contact with and penetrate dermal lymph vessels.17,33–35 In our study, the other factor that remained a predictor of locoregional cutaneous metastases after multivariate analysis was the location of the primary tumor on the head or neck, on the lower limbs, or, in particular, in acral sites. This finding is consistent with reports from previous studies, although the reason why locoregional cutaneous metastases are more common in primary tumors on the lower limbs has not yet been fully clarified. Some authors have suggested that it is due to the greater size and length of the lymph vessels that drain the legs. Another possible explanation is that, because of gravity, the lower limbs have greater lymphatic stasis, which would make the lymph vessel walls more permeable to neoplastic cells and allow those cells to persist as locoregional disease. Ours is the first study to describe a higher risk of locoregional cutaneous metastases in patients with a primary tumor located on the head or neck. Further research is needed to clarify this association.
In conclusion, we found 2 of the variables assessed in this study to be independent risk factors for locoregional cutaneous metastases. The factor most strongly associated with the development of locoregional cutaneous metastases was Breslow thickness, followed by location of the primary tumor. These findings could be useful in the design of future guidelines for the monitoring and management of melanoma. Because the findings of this study are based on retrospective data, larger patient series—preferably prospective—are needed to validate our results.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Messeguer F, et al. Factores de riesgo para el desarrollo de metástasis cutáneas locorregionales como forma única de recaída en los pacientes con melanoma. Actas Dermosifiliogr.2013;104:53-60.