Atopic dermatitis (AD) is the most frequent inflammatory dermatosis. Up to 20% of patients have moderate to severe disease. Of these, 20%–43% may experience ocular symptoms, including conjunctivitis, keratitis, and blepharitis.1
Dupilumab is the first monoclonal antibody approved for the treatment of moderate to severe AD. It binds to the common alfa subunit of interleukins (IL) 4 and IL-13, leading to a reduction in Th2-mediated inflammation and an improvement in epidermal barrier function. Although it is expected that control of AD could also improve ocular manifestations, in clinical trials, IL-4/IL-13 blockade with dupilumab is associated with a greater frequency of ocular manifestations than observed in the control group, which received placebo (incidence of 5%–28%, according to dose, compared with 1%–8%, respectively).2–4
Two basic clinical patterns of ocular involvement associated with the use of dupilumab have been reported:
- •
Nonspecific conjunctivitis, associated with dots on the cornea in the lower third and evaporative dry eye (lacrimal secretion is quantitatively normal but of a lower quality). This form can respond to measures to hydrate the ocular surface.
- •
Follicular conjunctivitis, more specific, generally bilateral and of a mild to moderate severity, associated with pruritus, burning sensation, and increased tear production. It is characteristic of limbal hyperemia and can be accompanied by blepharitis. This form is refractory to humectants and requires topical anti-inflammatory treatment.5
The cause of ocular manifestations associated with dupilumab is unknown, although the mechanism could be inflammatory and specific to AD, given that administration in patients with asthma or nasal polyposis has not been associated with an increased risk of conjunctivitis. The risk factors for its development include severe AD, personal history of conjunctivitis, and low serum levels of dupilumab.6 It is postulated that inhibition of IL-4/IL-13 itself could increase the activity of OX40L—which participates in the development of atopic keratoconjunctivitis—and also reduce the number of intraepithelial goblet cells; this would explain the lower proportion of mucin observed, in turn impacting tear quality.5,6
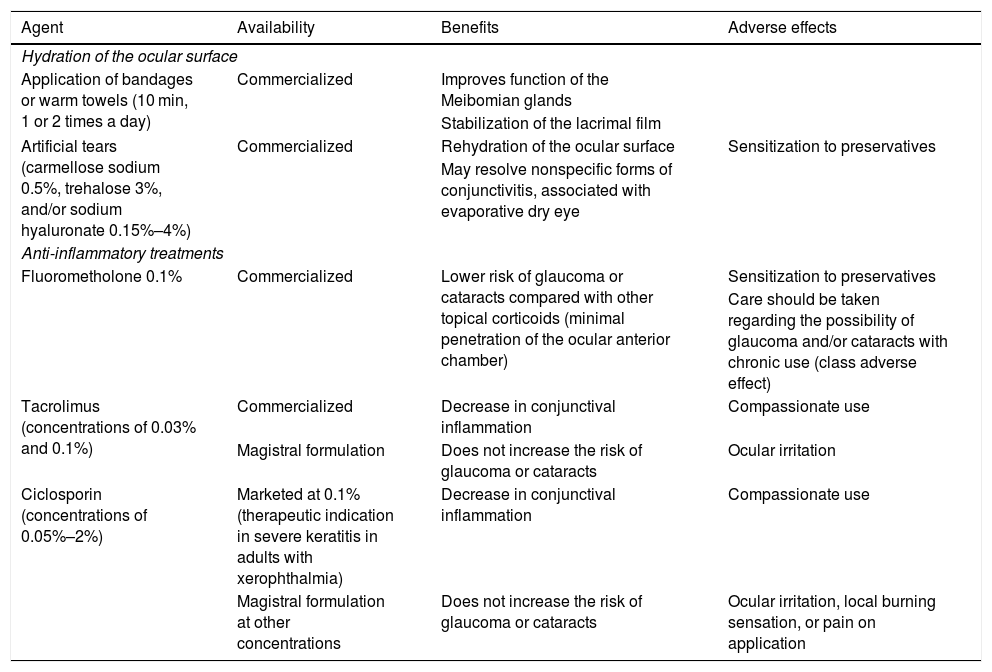
For diagnosis of dupilumab-associated conjunctivitis, it is essential to obtain a detailed clinical history, and findings of the eye examination (exudate, pruritus, and concurrent symptoms) and slit lamp assessment, to be performed by ophthalmologists. Differential diagnosis with other forms of conjunctivitis is essential, particularly, infectious forms.1 The therapeutic recommendations are presented in Table 1.5
Therapeutic Recommendations for Management of Dupilumab-Associated Conjunctivitis.
| Agent | Availability | Benefits | Adverse effects |
|---|---|---|---|
| Hydration of the ocular surface | |||
| Application of bandages or warm towels (10 min, 1 or 2 times a day) | Commercialized | Improves function of the Meibomian glands | |
| Stabilization of the lacrimal film | |||
| Artificial tears (carmellose sodium 0.5%, trehalose 3%, and/or sodium hyaluronate 0.15%–4%) | Commercialized | Rehydration of the ocular surface | Sensitization to preservatives |
| May resolve nonspecific forms of conjunctivitis, associated with evaporative dry eye | |||
| Anti-inflammatory treatments | |||
| Fluorometholone 0.1% | Commercialized | Lower risk of glaucoma or cataracts compared with other topical corticoids (minimal penetration of the ocular anterior chamber) | Sensitization to preservatives |
| Care should be taken regarding the possibility of glaucoma and/or cataracts with chronic use (class adverse effect) | |||
| Tacrolimus (concentrations of 0.03% and 0.1%) | Commercialized | Decrease in conjunctival inflammation | Compassionate use |
| Magistral formulation | Does not increase the risk of glaucoma or cataracts | Ocular irritation | |
| Ciclosporin (concentrations of 0.05%–2%) | Marketed at 0.1% (therapeutic indication in severe keratitis in adults with xerophthalmia) | Decrease in conjunctival inflammation | Compassionate use |
| Magistral formulation at other concentrations | Does not increase the risk of glaucoma or cataracts | Ocular irritation, local burning sensation, or pain on application | |
Following approval of dupilumab in Europe, an increase in the number of patients with drug-related ocular manifestations could be expected. It would be desirable to raise awareness of this potential issue, and put in place measures for their prevention and management. Prior ophthalmological assessment is recommended before administration of the drug, along with active screening for this possible side effect, as well as the development of new therapeutic algorithms.
Conflicts ofinterestJ.M. Carrascosa has received speaker and consultant fees from Sanofi and fees as an investigator in clinical trials from Sanofi, Lilly, Leo-Pharma, Pfizer, and Amgen. M. Munera-Campos has participated in and received fees as an investigator in clinical trials from Lilly, Leo-Pharma, and Pfizer.
Please cite this article as: Munera-Campos M, Carrascosa JM. FR-Conjuntivitis asociada a dupilumab en dermatitis atópica. Actas Dermosifiliogr. 2020;111:872–873.