No information is currently available on whether the available clinical practice guidelines on the management of atopic dermatitis are known or being applied in Spain. The aim of this study was to improve the care of patients with atopic dermatitis by developing a set of quality indicators based on existing clinical practice guidelines. Relevant clinical practice guidelines identified through a literature search were submitted to a panel of 11 specialists, who selected the highest quality guidelines using the AGREE (Appraisal of Guidelines for Research & Evaluation) II instrument. The panel then defined a subset of the recommendations supported by a high level of evidence and proposed a health care quality indicator for each one together with a standard for measuring degree of adherence. Consensus was achieved on 21 of the 150 proposed indicators using the modified Delphi method. The aim of implementing the indicators that achieved consensus in this study is to standardize the actions of health professionals providing care for patients with atopic dermatitis and ultimately to improve the quality of the care delivered.
El grado de conocimiento y aplicación de las guías de práctica clínica sobre el manejo de la dermatitis atópica son desconocidos en nuestro entorno. El objetivo de este estudio es elaborar indicadores de calidad basados en las guías de práctica clínica existentes, para mejorar la atención de los pacientes. Tras una búsqueda bibliográfica de guías de práctica clínica, un grupo de 11 panelistas seleccionó las de mayor calidad mediante el instrumento AGREE II. Posteriormente se extrajeron recomendaciones con alto nivel de evidencia y propusieron un indicador de calidad asistencial asociado a un estándar para medir el grado de cumplimiento de cada recomendación. De los 150 indicadores propuestos, se obtuvo consenso en 21 de ellos tras la realización del método Delphi modificado. La implementación de los indicadores consensuados en este estudio pretende estandarizar las actuaciones de los profesionales sanitarios para mejorar la calidad asistencial de los pacientes con dermatitis atópica.
Atopic dermatitis (AD) is a chronic inflammatory disease that occurs in episodes and is characterized by very severe pruritus and marked xerosis.1 This disease may affect patients of any age, although it is more frequent in childhood, with a prevalence of up to 20%.1 It has a high impact on the quality of life of patients and family members.2 In 2010, the World Health Organization placed AD first among dermatologic diseases with the greatest effect on quality of life, with respect to years of life adjusted for disability and years spent with the disease.3 This disease also involves major health care, occupational and economic costs.
Many clinical practice guidelines (CPG) on the management of this disease exist but we do not know whether these guidelines are known and applied in our setting. With the aim of improving the care of patients with AD, the Spanish Academy of Dermatology and Venereology (AEDV) thought it useful to create quality indicators based on existing CPGs, which can serve as a reference and be used to measure the quality of care in different centers.
Materials and MethodsThis AEDV consensus statement was an initiative of the AEDV Board of Directors and was managed and funded by the Piel Sana Foundation.
The coordinators of the Contact Dermatitis and Skin Allergy (GEIDAC) and Pediatric Dermatology (GEDP) groups invited 4 members of each group considered experts in atopic dermatitis to take part in this study. The coordinator of the Dermatology Group of the Catalan Society for Family and Community Medicine (CAMFiC), a pediatrician, and a representative of the Spanish Atopic Dermatitis Association were also included in the drafting of this consensus statement. A total of 11 panelists were selected, who formed part of a multidisciplinary team. All of them declared their conflicts of interest. Participation of panelists with significant conflicts of interest in the topics in question was prevented.
First, a search was made for CPGs on AD in data-gathering bodies, such as the National Guideline Clearinghouse, Epistemonikos, drafting bodies, such as the National Institute for Clinical Excellence, methodology centers, such as the Guideline International Network, Guía Salud, and general databases, such as Medline and Embase. The search was performed in October 2017. The search strategy is attached as Appendix, Supplementary material (search strategy).
Guidelines that were more than 6 years old, those that did not form part of the clinical-dermatologic setting and those of lesser quality were excluded. Evaluation of the quality of each guideline was carried out by 2 panelists, independent in each guideline, using the AGREE II instrument4 (Appendix B available evaluations in Supplementary material, Table 1).
Two independent panelists on each guide extracted recommendations for the management of AD from each CPG. Only recommendations made with a level of evidence based on clinical trials or systematic reviews of clinical trials in the area of treatment and systematic reviews of quality observational studies in other areas were included. Furthermore, the panelists proposed an indicator associated with a standard for measuring the level of compliance with each recommendation. The standard for each indicator was established by consensus among the panelists, as their values were not found in the literature.
The next phase consisted of a search for consensus of the proposed indicators, taking into account their relevance and feasibility, using a 2-round modified Delphi method. During the consensus process, each panelist evaluated the relevance and feasibility of each proposed indicator. Relevance was deemed to refer to the impact of the indicator on the quality of care and feasibility to the possibility of measuring or obtaining the indicator from the available data (generally the medical records). Each indicator was assigned a score on a scale from 1 (not relevant/not feasible) to 9 (highly relevant/highly feasible). In the first round, the panelists were also able to suggest indicators that they considered relevant and feasible, and whose content was not adequately reflected in the indicators previously extracted from the CPGs. In the second round, the participants were able to see their own scores and the means generated by all the panelists in the first phase of the method, and then re-evaluate the indicators if they deemed it necessary. After completing the 2 rounds, the indicators were classified, based on their scores, as follows: indicator with consensus for, if the median and the mode were greater than 7; indicator with consensus against, if the median and mode were less than 2; indicator with consensus neither for nor against, if the median and the mod were greater than 3 and less than 6; an indicator was considered to be without consensus when neither the median nor the mode were within the aforementioned ranges. Indicators without consensus or with a consensus against were ruled out. All the approved indicators were reviewed in a final meeting to ensure the consistency, proportionality in topics, and faithfulness to the source documents.
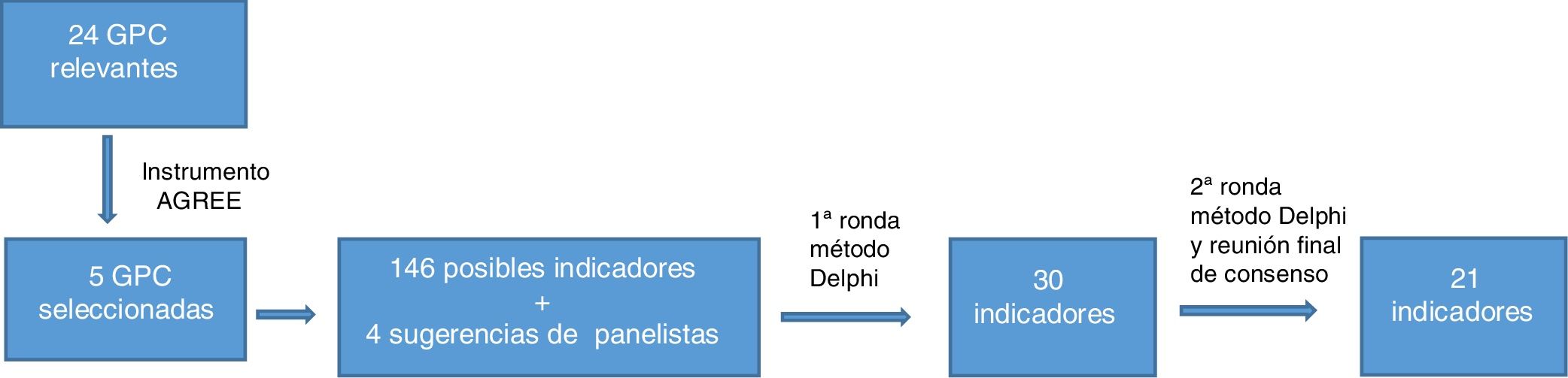
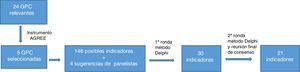
ResultsA total of 24 guidelines were found following the bibliographic search. Of these, 5 CPGs were selected after using the AGREE II instrument. A total of 146 possible indicators were developed based on the recommendations extracted from the guidelines and 4 panelist suggestions were added. After grouping or unifying the indicators that overlapped and eliminating those with controversial evidence, 30 indicators went through to the second round. The modified Delphi method provided consensus on 21 indicators (Fig. 1). Of these, 17 were process indicators, 3 were structural indicators, and 1 was a result indicator. Moreover, of the 21 quality indicators selected, 8 refer to topical treatments, 5 to systemic treatments, 3 to phototherapy, 3 to patient education, and 2 to diagnosis of the disease.
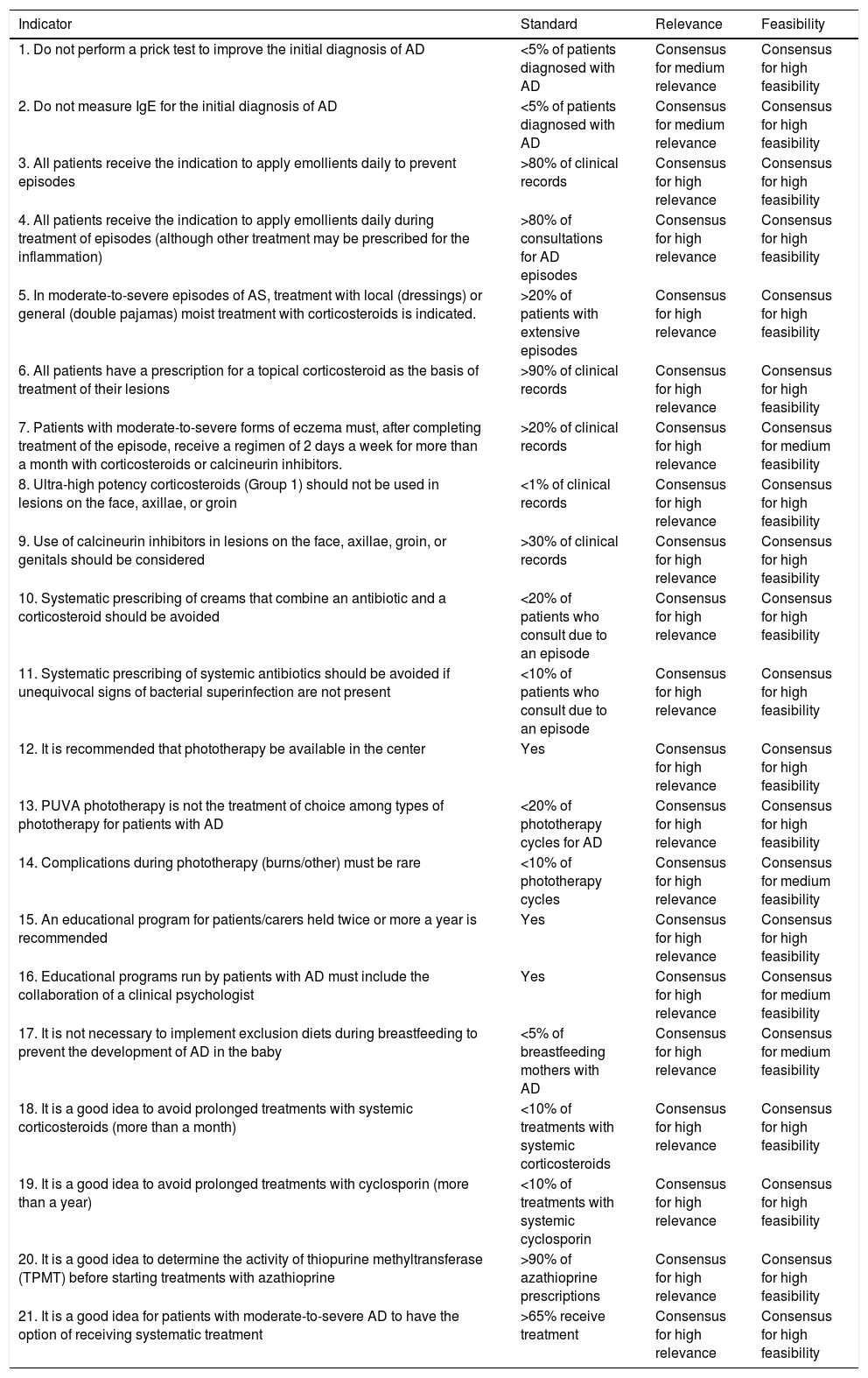
The remaining indicators, recommended standards, and the data on the degree of consensus are shown in Table 1. Detailed information on the indicators, including the literature on which they are based, is available in Table 2 of Appendix B, Supplementary material.
Proposed Quality-of-Care Indicators in Atopic Dermatitis These situations can be corrected by using indicators of quality of care in AD.
| Indicator | Standard | Relevance | Feasibility |
|---|---|---|---|
| 1. Do not perform a prick test to improve the initial diagnosis of AD | <5% of patients diagnosed with AD | Consensus for medium relevance | Consensus for high feasibility |
| 2. Do not measure IgE for the initial diagnosis of AD | <5% of patients diagnosed with AD | Consensus for medium relevance | Consensus for high feasibility |
| 3. All patients receive the indication to apply emollients daily to prevent episodes | >80% of clinical records | Consensus for high relevance | Consensus for high feasibility |
| 4. All patients receive the indication to apply emollients daily during treatment of episodes (although other treatment may be prescribed for the inflammation) | >80% of consultations for AD episodes | Consensus for high relevance | Consensus for high feasibility |
| 5. In moderate-to-severe episodes of AS, treatment with local (dressings) or general (double pajamas) moist treatment with corticosteroids is indicated. | >20% of patients with extensive episodes | Consensus for high relevance | Consensus for high feasibility |
| 6. All patients have a prescription for a topical corticosteroid as the basis of treatment of their lesions | >90% of clinical records | Consensus for high relevance | Consensus for high feasibility |
| 7. Patients with moderate-to-severe forms of eczema must, after completing treatment of the episode, receive a regimen of 2 days a week for more than a month with corticosteroids or calcineurin inhibitors. | >20% of clinical records | Consensus for high relevance | Consensus for medium feasibility |
| 8. Ultra-high potency corticosteroids (Group 1) should not be used in lesions on the face, axillae, or groin | <1% of clinical records | Consensus for high relevance | Consensus for high feasibility |
| 9. Use of calcineurin inhibitors in lesions on the face, axillae, groin, or genitals should be considered | >30% of clinical records | Consensus for high relevance | Consensus for high feasibility |
| 10. Systematic prescribing of creams that combine an antibiotic and a corticosteroid should be avoided | <20% of patients who consult due to an episode | Consensus for high relevance | Consensus for high feasibility |
| 11. Systematic prescribing of systemic antibiotics should be avoided if unequivocal signs of bacterial superinfection are not present | <10% of patients who consult due to an episode | Consensus for high relevance | Consensus for high feasibility |
| 12. It is recommended that phototherapy be available in the center | Yes | Consensus for high relevance | Consensus for high feasibility |
| 13. PUVA phototherapy is not the treatment of choice among types of phototherapy for patients with AD | <20% of phototherapy cycles for AD | Consensus for high relevance | Consensus for high feasibility |
| 14. Complications during phototherapy (burns/other) must be rare | <10% of phototherapy cycles | Consensus for high relevance | Consensus for medium feasibility |
| 15. An educational program for patients/carers held twice or more a year is recommended | Yes | Consensus for high relevance | Consensus for high feasibility |
| 16. Educational programs run by patients with AD must include the collaboration of a clinical psychologist | Yes | Consensus for high relevance | Consensus for medium feasibility |
| 17. It is not necessary to implement exclusion diets during breastfeeding to prevent the development of AD in the baby | <5% of breastfeeding mothers with AD | Consensus for high relevance | Consensus for medium feasibility |
| 18. It is a good idea to avoid prolonged treatments with systemic corticosteroids (more than a month) | <10% of treatments with systemic corticosteroids | Consensus for high relevance | Consensus for high feasibility |
| 19. It is a good idea to avoid prolonged treatments with cyclosporin (more than a year) | <10% of treatments with systemic cyclosporin | Consensus for high relevance | Consensus for high feasibility |
| 20. It is a good idea to determine the activity of thiopurine methyltransferase (TPMT) before starting treatments with azathioprine | >90% of azathioprine prescriptions | Consensus for high relevance | Consensus for high feasibility |
| 21. It is a good idea for patients with moderate-to-severe AD to have the option of receiving systematic treatment | >65% receive treatment | Consensus for high relevance | Consensus for high feasibility |
Abbreviations: AD indicates atopic dermatitis.
This study proposes a set of indicators for evaluating the quality of dermatologic care of patients with AD. The selected indicators cover different areas of the disease, such as initial diagnosis, patient education, and management with different treatments.
Except for the 3 indicators proposed by the panel of experts, all the others are based on recommendations with a high level of scientific evidence, made in national and international guidelines. The resulting quality indicators are therefore valid in our setting but may also be of interest in other countries with similar availability of treatments and infrastructure.
It should be noted that the quality indicators obtained do not reflect all the treatment options available in AD. This limitation is due to the fact that some therapies are not shown in the guidelines (such as dupilumab, as it is a recent drug), their level of evidence was not sufficient for them to be selected as indicators (such as mofetil mycophenolate and methotrexate), or consensus in favor of their relevance and feasibility was not achieved (such as alitretinoin and interferon gamma).
The recommendations made in the different CPGs regarding systemic treatments in AD presented major discrepancies. The different systems for classifying levels of evidence and degrees of recommendation used by the different guidelines reviewed were taken into account.5–14 Most systemic therapies presented different levels of evidence and some therapies, such as antihistamines or immune therapy, showed opposing efficacy results. Indicators on treatments with opposing recommendations were avoided and those that presented different levels of evidence were subjected to consensus.
Few clinical trials compare the different systemic treatments in AD. Thus, recommendations were not found with a high level of evidence on the choice of a systemic treatment compared to other treatments. Although the only immunosuppressant indicated for AD, according to the technical data sheet, is cyclosporin, considerable experience exists and good results have been achieved with the use of others such as azathioprine, and quality indicators on these forms of treatment have therefore been included.
The proposed indicators should be complied with in at least a certain percentage of patients in order to improve patient care. A standard has therefore been proposed for each indicator. In none of the cases is the proposed standard 0% or 100%, as flexibility must exist in order to adapt to special situations.
The strength of the work lies in the use of standardized and validated methodology; the Delphi method is a systematic consensus technique that is widely used in developing quality-of-care indicators.15 Many studies in the field of health care have been published that use the simple or modified Delphi method to develop and validate quality indicators.16–18 The Canadian Dermatology Association recently published a consensus statement on the evaluation and management of adult patients with moderate-to-severe AD.19 The number of panelists taking part in the Canadian study was greater than in our study. However, the Canadian study is composed only of dermatologists and allergologists, whereas the Spanish team is multidisciplinary, with health care professionals from different specialisms and with patient participation. The experience of the panelists in different areas of AD has made it possible to achieve a comprehensive evaluation of the indicators proposed in our study, with different perspectives. Moreover, the Canadian study is focuses on an adult population with moderate-to-severe AD, whereas most of our proposed indicators are applicable in other clinical situations, such as a pediatric population or in different degrees of severity of the disease.
AS is a common disease that must be recognized and treated appropriately by the different health care professionals who take part in the care of these patients. It is also important to correctly educate the patients so that they can take a proactive approach to their disease. However, the disease is often incorrectly managed because unnecessary additional tests are performed, contradictory recommendations are made by different specialists, treatment decisions are based on the experience of the clinician, the treatments are not shown in the clinical records, infrastructure is lacking for carrying out treatments such as phototherapy, and suboptimal educational programs are implemented. These situations can be corrected by using indicators of quality of care in AD. Implementation of the consensus indicators in this study aims to standardize the actions of health care professionals, based on high levels of evidence, to improve the quality of care, which effects the health of patients with atopic dermatitis.
FundingThis study was promoted and funded by the AEDV Piel Sana Foundation. No pharmaceutical entity has provided financial aid or participated in drawing up or carrying out the project.
Conflicts of InterestThe authors declare the following potential conflicts of interest with regard to this study: IB has obtained funding from Novartis and Pierre Fabre for conferences, educational programs and courses; JFS has participated as a speaker for Sanofi, Novartis, Janssen, and MSD, and as a consultant for Sanofi, Novartis, and Viñas; MIM has worked with the EDA and has received funding from Italfarmaco for conferences and courses; IP has received funding from Novartis, Janssen, ISDIN, Avène, Lilly, Leo Pharma, Almirall, and Pierre Fabre for conferences and courses; MS has received funding from ISDIN for courses and conferences, has received fees as a speaker for ISDIN, Lilly, and Novartis, has been a consultant for ISDIN, and has been a speaker at and chair of the scientific and organizational committee of the 1st and 2nd CAMFiC Skin Disease Conference, sponsored by Leo, Novartis, ISDIN, Janssen; the Spanish Atopic Dermatitis Association has received funding for conferences with patients, web development and social media management from the following: Sanofi, Leo Pharma, Leti, and Expanscience, and has taken part in conferences with Avène and Pfizer; FJMM has been a speaker for Novartis, Leo Pharma, and Janssen, a consultant for Novartis, and has received funding for conferences and courses from Novartis, Janssen, Leo Pharma, Sanofi, and Almirall. The other authors declare that they have no conflicts of interest.
This project was made possible by the collaboration of the members of AEDV. The idea for the study arose from the AEDV Working Groups such as the Spanish Contact Dermatitis and Skin Allergy Research Group (GEIDAC) and the Spanish Pediatric Dermatology Group (GEDP).
Please cite this article as: Poveda-Montoyo I, García-Doval I, Descalzo MA, Betlloch-Mas I, Miquel-Miquel FJ, Serrano-Manzano M, et al. Indicadores de calidad en la atención dermatológica a pacientes con dermatitis atópica. Documento de consenso de la Academia Española de Dermatología y Venereología. Actas Dermosifiliogr. 2020;111:567–573.