Pyoderma gangrenosum is a condition that is included among the neutrophilic dermatoses. Given its low incidence, few studies have addressed its epidemiology or treatment.
ObjectiveTo describe the epidemiological and clinical characteristics of patients with pyoderma gangrenosum along with our experience of treating the condition in a referral hospital in Malaga, Spain.
Material and methodsA retrospective, observational study was undertaken in the Department of Dermatology at Hospital Clínico Universitario Virgen de la Victoria in Malaga, Spain between January 2000 and December 2009 and included all patients diagnosed with pyoderma gangrenosum.
ResultsThe incidence of pyoderma gangrenosum in our reference population is 3.26 cases per million inhabitants per year. The most frequent concomitant systemic disease was ulcerative colitis (5 cases, 33%). In 4 patients with that disease, pyoderma gangrenosum appeared during a flare-up. In 80% of cases, patients were not referred to a dermatologist during the initial phase of pyoderma gangrenosum, and most referrals were from gastroenterology or general surgery (4 patients each, 52%).
ConclusionsPatients with pyoderma gangrenosum are often referred to dermatologists by other specialists after a varying period of time has elapsed without achieving an accurate diagnosis. In these patients, especially those between 20 and 40 years of age, it is essential to rule out concomitant disease. Adalimumab is a good treatment option for pyoderma gangrenosum.
El pioderma gangrenoso (PG) es un proceso incluido en el grupo de las denominadas dermatosis neutrofílicas, del que existen pocos trabajos epidemiológicos y de abordaje terapéutico en la bibliografía dada su relativa escasa incidencia.
ObjetivoDescribir las características epidemiológicas y clínicas y exponer nuestra experiencia terapéutica en los pacientes con PG de un hospital de segundo nivel de Málaga (España).
Material y métodosEl estudio observacional y retrospectivo incluyó todos los pacientes diagnosticados de PG en el Servicio de Dermatología del Hospital Clínico Universitario Virgen de la Victoria (Málaga) en un periodo de 10 años, comprendido entre enero de 2000 y diciembre de 2009.
ResultadosLa incidencia del PG en nuestra población de referencia es de 3,26 casos por millón de habitantes y año. La enfermedad sistémica asociada con mayor frecuencia a la aparición del PG fue la colitis ulcerosa (5 casos, 33%). En 4 pacientes con colitis ulcerosa el PG apareció durante un brote de la enfermedad. El 80% de los pacientes no fueron derivados a Dermatología en la fase inicial del PG, siendo los servicios con más derivaciones Digestivo y Cirugía general, con 4 pacientes cada uno (52%).
ConclusionesEl PG a menudo llega al dermatólogo remitido por otros especialistas tras un tiempo variable sin diagnóstico correcto. Ante este proceso es básico descartar la existencia de alguna patología asociada, especialmente en pacientes entre 20 y 40 años. Adalimumab es una buena opción terapéutica en el tratamiento del PG.
Pyoderma gangrenosum (PG) is a skin condition characterized by the presence of single or multiple erythematous pustules that progress rapidly to form necrotic ulcers with a depressed violaceous border.1 The incidence of PG is estimated at 2 to 3 cases per million inhabitants per year.1–4 It is most frequent in individuals aged between 30 and 50 years and is slightly more common in women.1–4 Half of all cases are associated with systemic disease, the most frequent being inflammatory bowel disease and seronegative arthritis, followed by lymphoproliferative syndromes and paraproteinemias.1–4 There are 4 clinical forms of PG: ulcerative (classic form), bullous, pustulous, and vegetative (superficial granulomatous form).1–4 It is usually treated with immunosuppressant drugs, generally corticosteroids or ciclosporin.4
Due to the relatively low incidence of PG, only limited information is available on its epidemiology. Notably, no reports have been published describing clinical findings and treatment response in Spanish patients. Furthermore, almost no clinical trials are available that would allow an evidence-based treatment algorithm to be established.4
The aim of this study was to investigate the clinical and epidemiologic characteristics and the treatments used in a series of patients with PG seen over a 10-year period in the Department of Dermatology at Hospital Clínico Virgen de la Victoria in Malaga, Spain, and to compare the findings with those described in other regions.
Patients and MethodsA retrospective, observational study was undertaken in the Department of Dermatology at Hospital Clínico Universitario Virgen de la Victoria in Málaga, Spain between January 2000 and December 2009 and included all patients diagnosed with PG during that period. The health care area covered by the hospital serves a population of 460 000.
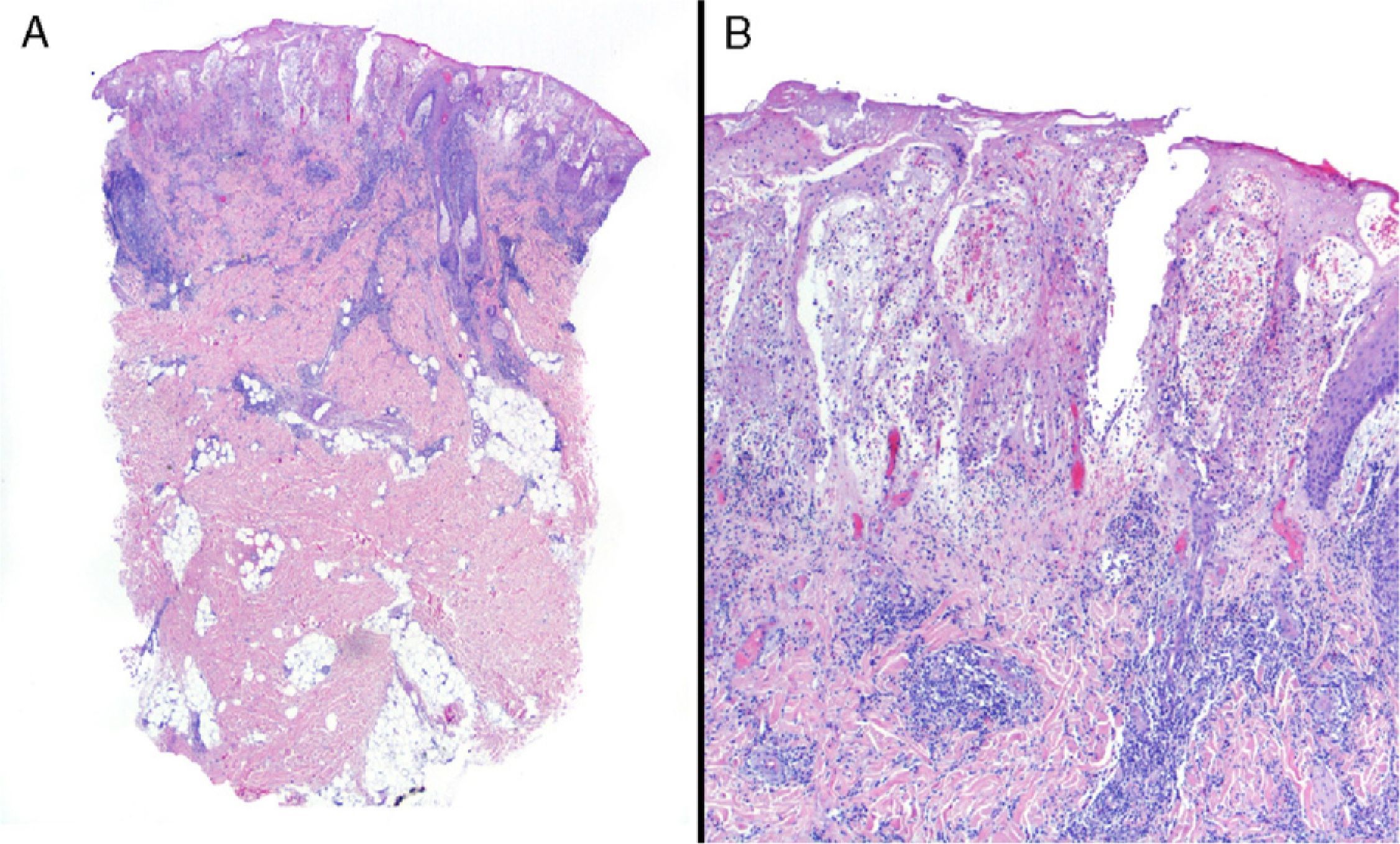
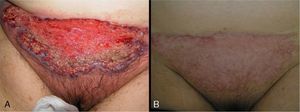
Data were collected by review of patient records and pathology reports. In all cases included in the study, diagnosis was based on clinical suspicion and histopathology to establish compatibility of the diagnosis and rule out infections, tumors, or other conditions such as vasculitis (Fig. 1).
The following variables were recorded: sex, age, time since appearance of symptoms, source of referral, and presence or absence of systemic disease. Treatment was also recorded, along with treatment response over a minimum follow-up period of 1 year.
Data were analyzed using SPSS 17.0 to obtain descriptive statistics (frequencies, percentages, and means) and between-group comparisons.
ResultsTable 1 shows the clinical characteristics of the patients. Of the 15 patients included in the study, 7 (43%) were men and 8 (57%) were women, which corresponds to a female to male ratio of 1.14 to 1. The mean (SD) age at diagnosis was 49.2 (17.4) years (range, 25-75 years) in the complete group.
Clinical Characteristics of the Patients.
| Patient | Clinical Presentation | Primary Referral | Sex | Age, y | Systemic Disease | Temporal Relationship With Systemic Disease | Treatment | Course |
| 1 | Ulcerative | Surgery | Female | 45 | No | – | Oral prednisone | No lesions after 2 months of treatment |
| 2 | Ulcerative | Surgery | Female | 71 | Ulcerative colitis | Preceding | Oral prednisone | No lesions after 2 months of treatment |
| 3 | Ulcerative | Vascular surgery | Male | 59 | No | – | Oral prednisone | No lesions after 2 months of treatment |
| 4 | Ulcerative | Gastroenterology | Male | 25 | Ulcerative colitis | Flare | Infliximab | No lesions after 6 weeks of treatment |
| 5 | Ulcerative | Dermatology | Female | 35 | Ankylosing spondylitis | None | Adalimumab/sulfasalazine | No lesions after 2 months of treatment |
| 6 | Ulcerative | Gastroenterology | Female | 28 | Ulcerative colitis | Flare | Oral prednisone | No lesions after 2 months of treatment |
| 7 | Peristomal | Surgery | Female | 35 | Crohn Disease | None | Adalimumab | No lesions after 1 month of treatment Required weekly dosing |
| 8 | Ulcerative | Gastroenterology | Female | 42 | Ulcerative colitis | Flare | Adalimumab | No lesions after 1 month of treatment |
| 9 | Ulcerative | Vascular surgery | Female | 61 | No | – | Oral prednisone | No lesions after 2 months of treatment |
| 10 | Peristomal | Vascular surgery | Male | 73 | Surgical intervention | – | Oral prednisone | No lesions after 2 months of treatment |
| 11 | Ulcerative | Gastroenterology | Male | 30 | Ulcerative colitis | Flare | Adalimumab | No lesions after 1 month of treatment |
| 12 | Peristomal | Surgery | Female | 75 | Surgical intervention | – | Triamcinolone dermojet injection | Improvement without complete resolution of lesions after 1 month of treatment |
| 13 | Ulcerative | Dermatology | Male | 60 | No | – | Oral prednisone | No lesions after 2 months of treatment |
| 14 | Ulcerative | Dermatology | Male | 34 | No | – | Oral prednisone | No lesions after 2 months of treatment |
| 15 | Peristomal | Urology | Male | 75 | Surgical intervention | – | Topical corticosteroids | Died during the first month of follow-up |
The mean age at diagnosis was 44.4 (18.7) years (range, 25-75 years) in men and 45.3 (15.4) years (range, 28-75 years) in women. Comparison of age groups revealed that 80% of patients were between 20 and 40 (6 patients) or between 60 and 80 years of age (6 patients); there were no patients younger than 25 or older than 75 years.
Ulcerative PG was the most common clinical form, accounting for 80% of the sample (12 cases). Peristomal PG was diagnosed in 3 patients (20%).
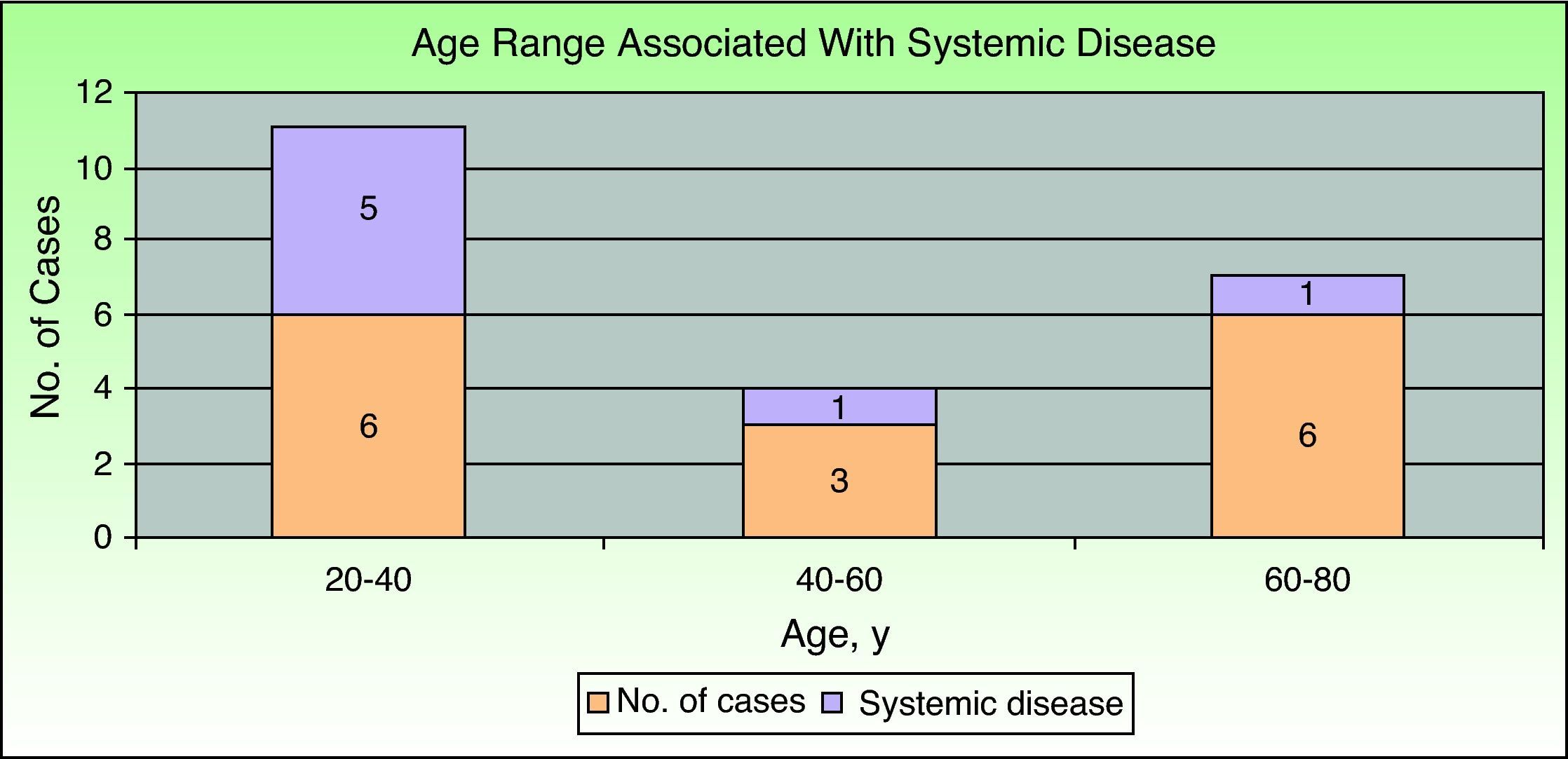
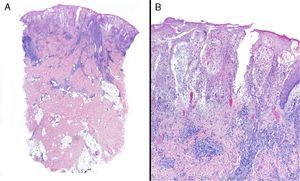
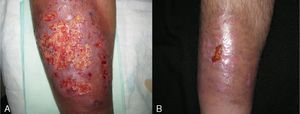
Inflammatory bowel disease was the most common associated systemic condition and was found in 40% of patients with PG (6 cases). Ulcerative colitis was present in 33% (5 cases) and Crohn disease in 7% (1 case). A surgical procedure had previously been carried out in the affected area in 20% of patients (3 cases), all of whom had peristomal PG. Only 1 patient had ankylosing spondylitis. The remaining 5 cases (33%) were idiopathic and there was no associated systemic disease. Consequently, if we exclude patients with prior surgical procedures, we find that 47% of patients (7 cases) had PG associated with a systemic condition. Comparison of those patients by age group showed that the majority (5 cases, 71.5%) were in the younger age group (20-40 years); 1 patient (14.25%) was in the middle age group (40-60) and another (14.25%) was in the older age group (60-80) (Fig. 2).
In patients with ulcerative colitis, the appearance of PG was linked to a flare of the disease, except in 1 case, where PG preceded the diagnosis of ulcerative colitis by 6 months. In contrast, no relationship between PG and the course of the systemic disease was observed in patients with Crohn disease or ankylosing spondylitis.
Only 20% of patients (3 cases) were referred directly to the dermatology department from primary care or the emergency department. The most common primary referral was to gastroenterology (4 patients, 26%) or general surgery (4 patients, 26%), followed by vascular surgery (3 cases, 20%) and urology (1 case, 8%).
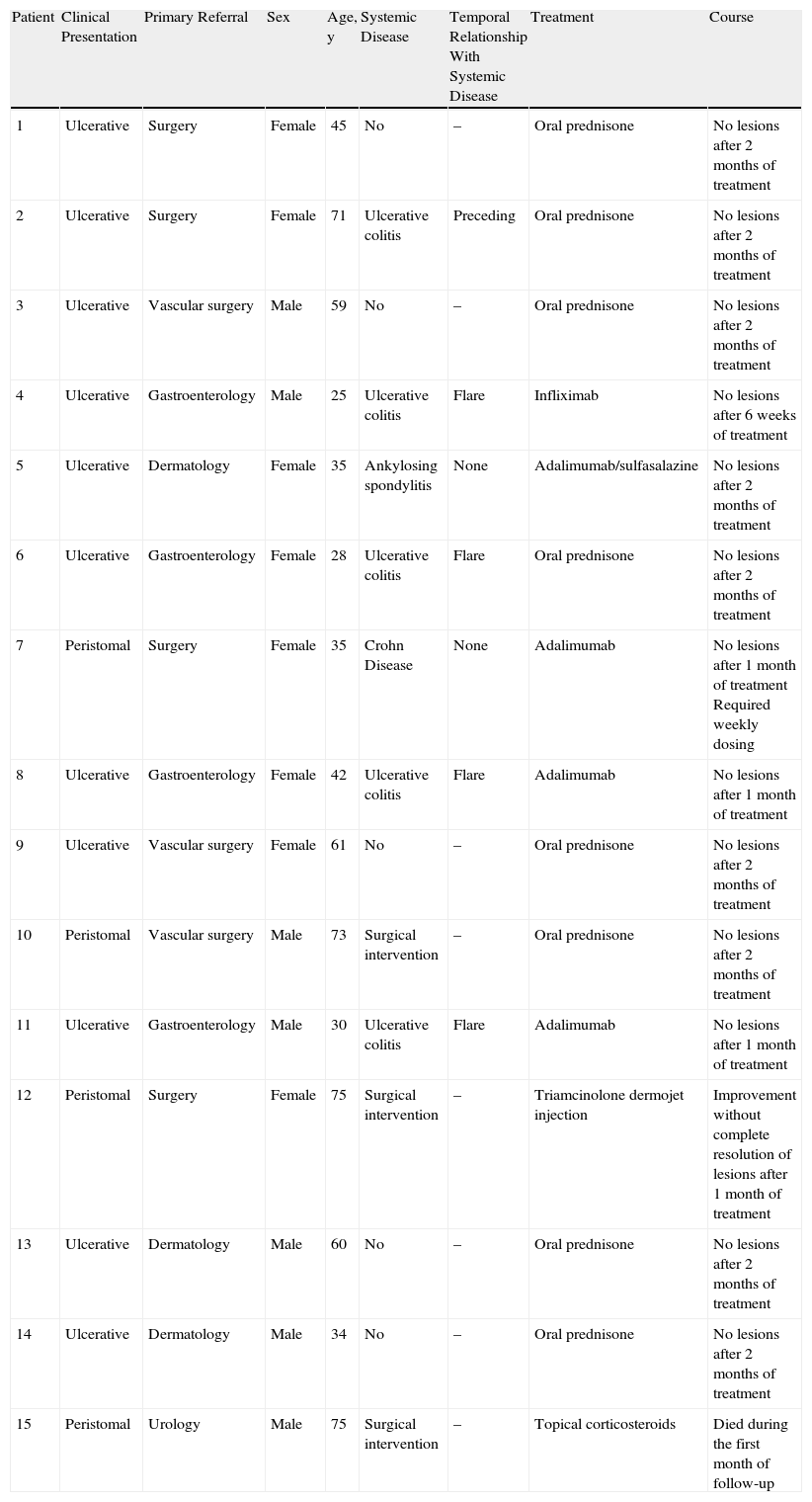
In patients with underlying systemic disease such as inflammatory bowel disease or ankylosing spondylitis, immunosuppressant drugs were chosen in consultation with the gastroenterology or rheumatology department, taking into consideration the activity of the disease and prior treatments. Table 2 shows the immunosuppressant drugs used for the treatment of PG and the associated level of evidence. Dressings were an important part of treatment in all patients in order to reduce infection and favor repair of destroyed tissue.
Level of Evidence for the Different Treatment Options.
| Treatment | Level of Evidence |
| Oral prednisone | 2 |
| Ciclosporin | 2 |
| Thalidomide | 2 |
| Methotrexate | 3 |
| Azathioprine | 3 |
| Infliximab | 1 |
| Adalimumab | 3 |
| Etanercept | 3 |
Source: Ruocco et al.3
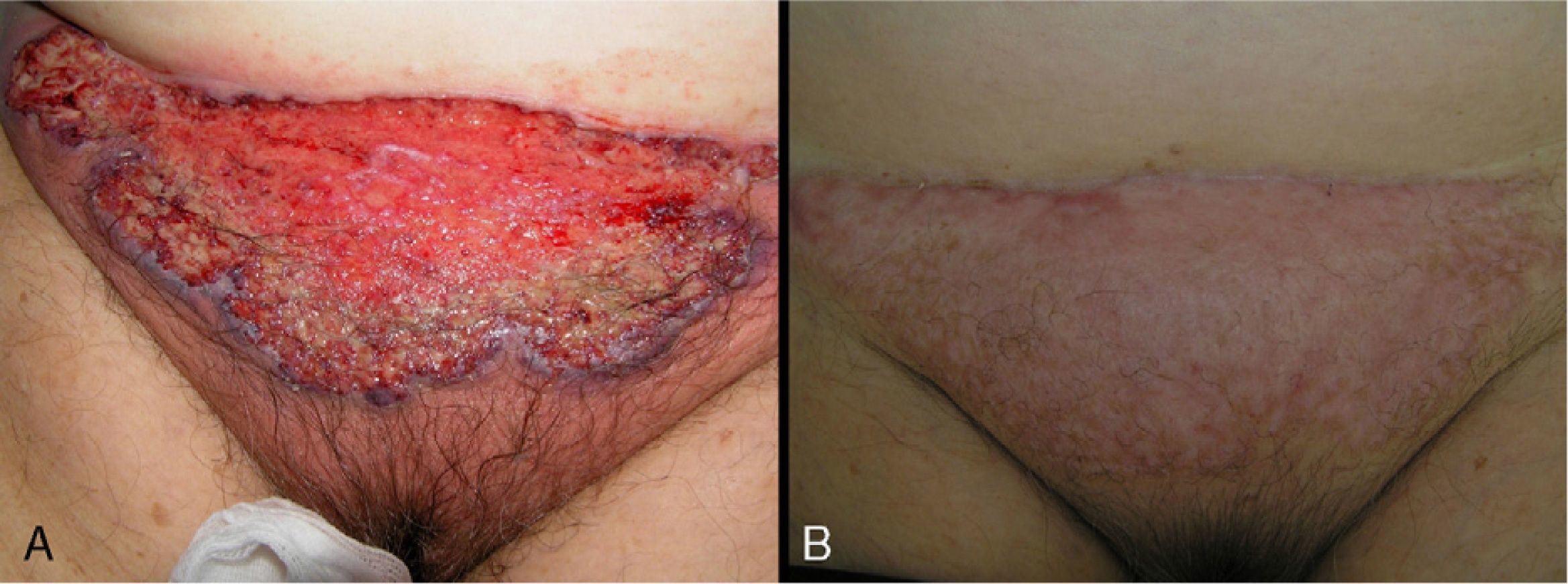
In 8 patients (53.3%), treatment was initiated with oral corticosteroids (prednisone 1mg/kg). Of those, only 2 had systemic disease (ulcerative colitis), and 1 had been diagnosed 6 months after the appearance of PG. All patients showed significant improvements within 2 weeks of initiation of oral corticosteroids. In all patients, ulcers were replaced by brownish plaques of scar tissue after 6 to 8 weeks of treatment (Fig. 3).
Two patients with ulcerative colitis were initially treated with infliximab. In one of those patients, infliximab was replaced with adalimumab after the patient experienced severe urticaria and hypotension. In the other patient, the lesions were completely resolved after 6 weeks of treatment with infliximab in combination with topical tacrolimus (0.03%).
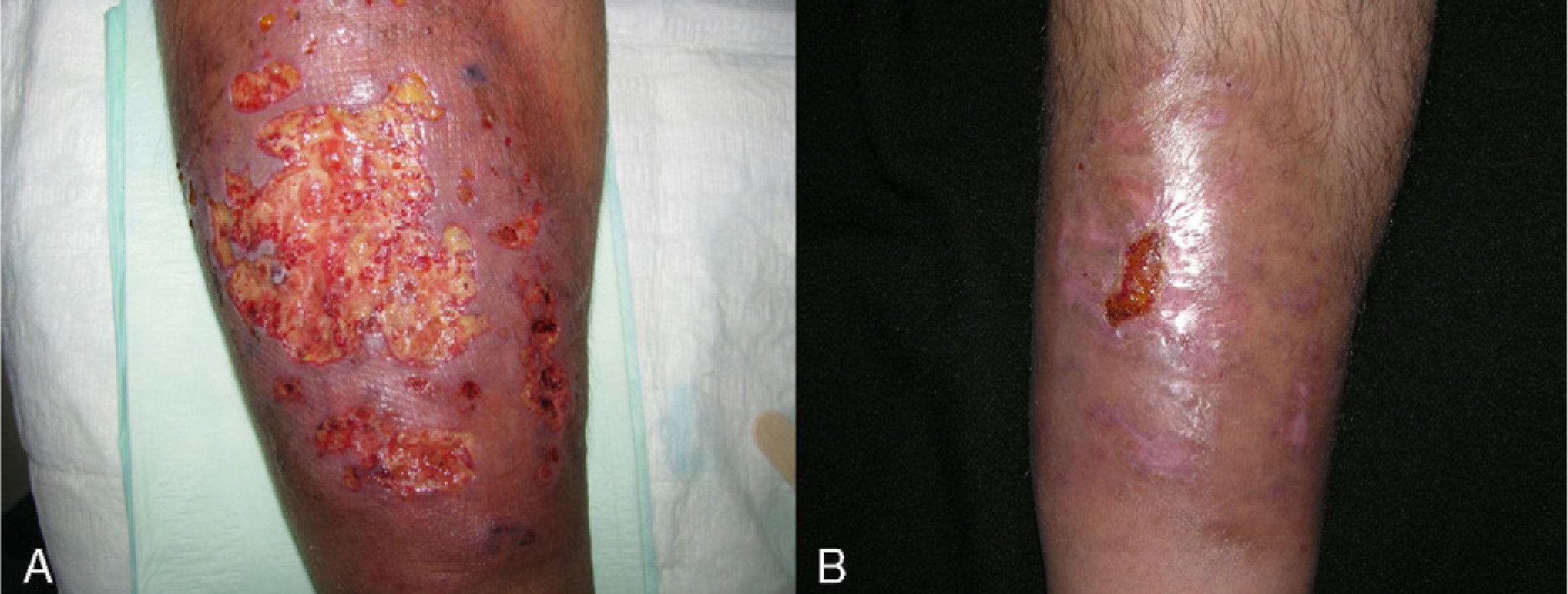
Adalimumab was used as first-line treatment in 4 patients (26.65%). Two of those patients had ulcerative colitis, 1 had ankylosing spondylitis, and 1 had Crohn disease. In the 2 patients with ulcerative colitis, complete resolution of PG was achieved 30 days after initiation of treatment, although adjuvant sulfasalazine (3g/d) was required in 1 patient. The patient with Crohn disease, who had additional concomitant disease (hypertension, insulin-dependent diabetes with diabetic nephropathy, and sulfasalazine intolerance due to headaches), required weekly dosing of adalimumab to control her intestinal disease and peristomal PG. The patient with ankylosing spondylitis required combined adalimumab and sulfasalazine when positive results were not obtained with adalimumab and oral prednisone or ciclosporin (Fig. 4).
Finally, 2 patients were treated with topical immunosuppressant drugs (0.05% clobetasol cream and dermojet injection of triamcinolone). One patient died 1 month after diagnosis of PG due to stage III carcinoma of the bladder, and the other showed significant improvement after 2 intralesional dermojet injections of triamcinolone (40mg/mL) 14 days apart.
DiscussionIn our study, only 15 patients with any type of PG were seen over a 10-year period (2000-2010). This low frequency represents a limitation when analyzing the results obtained. The incidence of PG in our reference population is 3.26 cases per million inhabitants per year. In our review of the literature, studies reported an estimated incidence of PG of between 2 and 3 cases per million inhabitants per year.1–4 The incidence observed in our population is therefore consistent with published reports. PG has traditionally been thought to be slightly more common in women.3,5–7 In our study, we observed a female to male ratio of 1.14 to 1. PG usually appears in patients aged between 30 and 50 years.3,6 The mean age at diagnosis in our study was 49.2 (17.4) years (range, 25–75 years). We nevertheless observed a peak incidence in patients aged between 60 and 80 years (6 cases, 40%).
Only 20% of cases (3 patients) were referred first to the dermatology department, indicating that dermatology plays a secondary role in the care of patients with PG. This may be because almost half of the patients included in our study had gastrointestinal disease, with PG coinciding with periods of worsening of the systemic disease. Nevertheless, these findings may also reflect a lack of knowledge of the condition among nondermatologists, who may confuse it with infections or consider it as secondary to vascular disease and therefore make an inappropriate referral.
In between 50% and 70% of cases, PG is associated with systemic disease.5,8,9 Ruocco et al3 report that idiopathic PG occurs in 25% to 50% of cases. In our study, 5 cases (33%) were not associated with systemic disease. We also did not observe any cases in which PG was associated with hematologic disease. In 3 cases (20%), PG was preceded by surgery: 1 sternotomy (aortic stenosis), 1 ileostomy (Crohn disease), and 1 nephrostomy (bladder carcinoma). The development of PG following surgery has been reported previously.10,11 The most common systemic disease in our patients was inflammatory bowel disease (40%; 5 cases of ulcerative colitis and 1 case of Crohn disease), and in most of those (4 cases with ulcerative colitis), PG developed during a disease flare. This finding differs from previous reports, since PG has always been described as independent of the activity of inflammatory bowel disease.1,6 Following initiation of immunosuppressant therapy in those 4 patients, control of both PG and ulcerative colitis was achieved.
None of the articles we found in the literature discussed a relationship between age and associated systemic disease in patients with PG. In our study, 5 cases (71.5% of patients classed as having associated systemic disease after exclusion of those with prior surgery) involved patients aged between 20 and 40 years. Due to the small number of patients included in the study (n=15), however, we were unable to assess a possible association between these 2 variables. It will be possible to examine such an association in future studies that pool data from multiple populations.
Given the low incidence of PG, there are no studies available to support the development of a standard treatment algorithm.4,12 Nevertheless, before choosing local or systemic therapy, the following patient characteristics should be taken into consideration: history, presence of concomitant systemic disease, depth and size of ulcers, and disease course.4
The use of dressings with hydrocolloids or hydrogels is particularly important, since they increase the production of collagen, improve the process of angiogenesis, optimize pain control, and reduce the likelihood of infection.13
Prednisone (1mg/kg) was the most frequently used immunosuppressant drug (8 patients, 53.3%). Complete resolution of the lesions was observed within 45 days of treatment initiation in all patients. In the literature, prednisone is considered the first-line systemic treatment, with a level of evidence of 2,4,5,12 and this is confirmed by our results. Ciclosporin can be used to lower the dose of corticosteroids, but its use should be restricted to idiopathic PG, since long-term ciclosporin therapy is not recommended.4
TNF inhibitors are used in patients with systemic disease and concomitant PG. Infliximab is the only biologic drug with demonstrated efficacy in double-blind placebo-controlled trials.4,5,14 We only used infliximab in 2 patients, and the drug had to be withdrawn in one of them due to a severe infusion reaction. The use of adalimumab has also been reported.3,12,15 The regimen used is that applicable to inflammatory bowel disease or ankylosing spondylitis. In inflammatory bowel disease, an initial dose of 80mg followed by 40mg every 2 weeks is used, whereas in ankylosing spondylitis, there is no loading dose and a dose of 40mg every 2 weeks is used. We prescribed adalimumab in 4 cases (26.65%); in 2 of those it was necessary to prescribe the drug in combination with sulfasalazine in order to obtain complete control of the associated systemic disease and resolution of the lesions. We did not observe adverse reactions with adalimumab.
Treatment with local immunosuppressants is indicated for superficial lesions or in patients with multiple diseases, in whom there is an unacceptable likelihood of adverse reactions to systemic therapy.4 Our experience with local immunosuppressants is limited. One of our patients died 1 month after diagnosis of peristomal PG and it was not possible to assess the response to application of clobetasol 0.05%; the other patient was lost to follow-up after 3 months, although partial improvement had been observed at 1 month following 2 dermojet injections of triamcinolone.
ConclusionsIn summary, PG is a rare condition in Spain. It affects similar numbers of men and women and is normally associated with systemic disease, particularly inflammatory bowel disease. All of these findings agree with the results reported in the literature. Notably, however, in our study there was a larger proportion of patients aged over 60 years than reported in the literature.
According to our findings, age younger than 40 years appears to be related to an increase in the probability of having an associated systemic disease in patients with PG. Nevertheless, further studies in a much larger sample of patients are required to confirm this hypothesis.
Although systemic corticosteroids continue to be the treatment of choice, we consider adalimumab to be a good alternative for the treatment of PG with concomitant systemic disease, given that it is effective, can be used in combination with other immunosuppressant drugs and dressings, is well tolerated, and has a favorable safety profile.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Suárez-Pérez JA, et al. Pioderma gangrenoso: Presentación de 15 casos y revisión de la literatura: Actas Dermosifiliogr.2012;103:120-126.