Scarce scientific evidence is available to define the precise effects that certain drugs might have on embryonic and fetal development if taken by pregnant women with psoriasis, given the ethical concerns that preclude enrolling such women in clinical trials. The little information on the use of biologics during gestation that has been published is based on retrospective and observational studies, and experience with these drugs in this context in psoriasis is still very limited. The literature seems to suggest that biologic therapy is safe during pregnancy, but there is no certainty. This detailed review of accumulated experience with biologic therapy during pregnancy relies mainly on descriptions of the management of other types of rheumatic disease, although the use of these agents in psoriasis is growing steadily.
Actualmente disponemos de escasa evidencia científica que defina el efecto exacto del uso de determinados fármacos sobre el desarrollo embrionario o fetal en gestantes con psoriasis debido a la ausencia de ensayos clínicos llevados a cabo en esta población por razones éticas. La información publicada respecto al uso de terapia biológica durante la gestación es escasa, y se basa en estudios retrospectivos y observacionales, y la experiencia de su uso en psoriasis, por el momento, es escasa. Los datos recogidos en la literatura parecen ofrecer una seguridad aceptable respecto al uso de terapia biológica durante la gestación; sin embargo no existe una evidencia absoluta. Esta revisión intenta describir con detalle la experiencia acumulada publicada en la literatura respecto al uso de terapia biológica en la gestación, aunque, como sabemos, la mayoría de los artículos se refieren a otro tipo de enfermedades reumatológicas, pero su uso en psoriasis crece progresivamente.
Both the US Food and Drug Administration (FDA) and the European Medicines Agency consider that it has not yet been demonstrated that biologic therapy is safe in the treatment of psoriasis during pregnancy, and stress the need for postmarketing surveillance. Biologic agents are classified as category B pregnancy drugs by the FDA. In other words, no apparent risk has been detected in animal reproduction studies, but well-controlled studies have not been conducted in pregnant women, and it is therefore not known whether biologics could cause fetal damage in humans.1 Evidence regarding the use of biologics during pregnancy and breastfeeding is classified as level III-IV, as it is based on information from descriptive nonexperimental studies, comparative studies, and case-control studies.2 While there is no certainty, experience accumulated in the literature on exposure to biologic therapy in pregnant women appears to indicate adequate safety, but the data must be interpreted with caution and multiple aspects need to be taken into account. Most of the data available are from pregnant women with rheumatoid arthritis and inflammatory bowel disease, and there has been very little experience with psoriasis.
Exposure to biologic therapy during pregnancy can be classified as:
- -
Unintentional, i.e. exposure at the time of conception or during the first trimester of pregnancy
- -
Intentional, i.e. exposure in women treated with biologics during pregnancy due to the refractory nature of their disease
The duration of treatment and exposure to biologics during pregnancy varies. According to most pregnancy registries, most women interrupt treatment as soon as their pregnancy is confirmed, generally in the first trimester.1 The course of each pregnancy also depends on multiple other factors, such as the disease, level of disease activity, and the presence of comorbidities, and this information is often missing from many publications.
This review on the use of biologic therapy in pregnant women with psoriasis draws on information from small case series, large retrospective studies, and registries managed by pharmaceutical companies and various international organizations and associations, including the Organization of Teratology Information Specialists (OTIS), which is a collaborative research group in the United States and Canada that collects data on the use of anti-tumor necrosis factor (TNF) agents during pregnancy.1 The 2 most important databases that provide information on exposure to infliximab during pregnancy are the FDA's Crohn's Therapy, Resource, Evaluation and Assessment Tool (TREAT) and the Infliximab Safety Database, managed by Centocor.3 The British Society for Rheumatology identified 35 pregnant women in a group of 11 473 women treated with anti-TNF agents. The Benefit Risk Management Worldwide Safety Database SCEPTRE,5 held by Johnson & Johnson, contains information on all reported cases of pregnancy in patients who were receiving infliximab before or after conception. Finally, the Spanish BIOBADASER registry holds information on 14 pregnancies in 13 women (of a total of 3550) with various rheumatologic diseases treated with biologic agents.6
Anti-TNF Biologic AgentsTNF is a multifunctional cytokine that regulates hormone synthesis, placental architecture, and embryo development during pregnancy. Elevated levels of circulating TNF have been associated with preeclampsia, abortion, and infertility in both human and animal models, and the cytokine is known to play an important role in hormone- and inflammation-mediated fetal loss.7,8 Two recently published observational cohort studies showed that anti-TNF agents were both effective and reliable in patients with a history of recurrent miscarriages and infertility.9,10 Regarding the effect of anti-TNF therapy on male fertility, it is known that TNF is produced by testicular germ cells and plays an important role in the regulation of spermatogenesis. TNF levels are generally low in seminal fluid, but tend to increase in certain inflammatory and infectious diseases, and pathologically elevated concentrations of TNF can disrupt the functional and genomic integrity of spermatozoa.11
TNF also has an important role in the development of the immune system, and consequently, exposure to anti-TNF agents in pregnant women could interfere with the development of the immune system in newborns. However, beyond transplacental passage, the possible long-term effects, if any, of exposure are currently unknown.12
In the following sections, we report in detail on the scientific evidence available on the use of each of the biologic agents used to treat psoriasis in pregnant women.
InfliximabInfliximab is a monoclonal antibody composed of a variable murine region and a constant human region. It binds with high specificity and affinity to the trimeric, dimeric, and monomeric forms of TNF, and additionally neutralizes transmembrane receptors and TNF-bearing cells. To date, information compiled from scientific publications and conference proceedings has been gathered on over 300 pregnancies in women treated with infliximab.13
Mahadevan et al.14 were the first authors to describe the intentional use of infliximab during pregnancy to induce and maintain remission of Crohn disease in a series of 10 patients; 8 of the patients were treated throughout pregnancy, while the other 2 were treated during the first trimester only. All the pregnancies resulted in live births and there was no record of congenital malformations or intrauterine growth restriction. There were 3 cases of premature birth and 1 of low birth weight.
Most of the data available on pregnancy course and outcomes in women treated with infliximab come from 2 large registries: the Infliximab Safety Database and the TREAT registry. Katz et al.15 analyzed the first database and identified 131 pregnant women who had been exposed to the drug. They were able to collect data on 96 patients (82 with Crohn disease, 8 with rheumatoid arthritis, 1 with ulcerative colitis, and 3 with an unknown diagnosis). Details of exposure time were available for 90 of the 96 patients: 29% had been exposed in the 3 months prior to conception and during the first trimester. Of the 96 pregnancies, 67% ended in live births, 15% in miscarriage, and 19% in therapeutic terminations. Among the 68 live births, there were 5 cases of fetal anomalies, including 1 case of intestinal malrotation (with evidence of additional intrauterine exposure to leflunomide, a known teratogen) and 1 case of tetralogy of Fallot. Nevertheless, these rates are similar to those observed in the general population of pregnant women and in pregnant women with Crohn disease not exposed to infliximab.
The TREAT registry is a prospective registry of patients with Crohn disease who have or have not been treated with infliximab. Lichtenstein et al.16 reviewed the data of 5807 patients and identified 36 pregnant women who had been exposed to the drug. They found no significant differences between exposed and unexposed women for either abortion rates (11% in exposed women vs 7.1% in unexposed women) or perinatal complications (8.3% in exposed women vs 7.1% in unexposed women).
Carter et al.17suggested a possible association between congenital anomalies that form part of the VACTERL spectrum and the use of anti-TNF therapy during pregnancy. The acronym VACTERL is used to describe the association between Vertebral abnormalities, Anal atresia (imperforate anus), Cardiac defect (mostly auricular and ventricular septum defects and tetralogy of Fallot), TracheoEsophageal fistula with esophageal atresia), Renal and/or radial abnormalities, and lower Limb abnormalities. It occurs in 1.6 of every 10 000 live births. Other TNF inhibitors, such as thalidomide and sodium valproate, have been linked to congenital abnormalities, some of which form part of the VACTERL association. On reviewing over 120 000 adverse events, Carter et al. identified 61 congenital anomalies in 41 children born to women taking TNF inhibitors. Twenty-four (59%) of the children had one or more congenital anomalies within the VACTERL spectrum, seven had 2 or more, and only one was diagnosed with true VACTERL. Fourteen of the mothers had taken etanercept and 10 had taken infliximab. The true significance of these results, however, is open to interpretation, as it is not known how many pregnant women were exposed. There were also methodological shortcomings with the collection of data: no information was given on the dose or time of exposure; the number of women exposed was not specified (which makes it difficult to estimate the magnitude of the associated risk); the anomalies described are very common in the general population; and in some cases, these conditions in isolation do not necessarily form part of the VACTERL association.18
In a recent publication, Crijns et al.19 found no statistically significant differences between the distribution of malformations in the VACTERL association from the series by Carter et al.17 and their distribution in the general population, based on information from the very large European population-based database of congenital malformations, EUROCAT, which contains records of congenital anomalies, including live and still births, since 1980.
Schnitzler et al.20 conducted an observational study that evaluated pregnancy outcomes in 212 women with inflammatory bowel disease treated with anti-TNF agents. They compared the outcomes of 42 pregnancies in which there had been direct exposure to anti-TNF therapy (infliximab in 35 cases and adalimumab in 7) with those of 23 pregnancies prior to a diagnosis of inflammatory bowel disease, 78 pregnancies prior to commencement of infliximab therapy, 53 pregnancies with indirect exposure to infliximab, and 56 pregnancies in healthy women. Thirty-two of the 42 pregnancies with direct exposure to anti-TNF agents resulted in live births, with a median gestational age of 38 weeks. There were 7 premature births and 6 cases of low birth weight. One boy born at week 33 weighing 1640 g died after 13 days due to necrotizing enterocolitis. There were 8 abortions (including 1 voluntary termination) in 7 women. There was also 1 case of trisomy 18 in the child of a mother treated with adalimumab. No significant differences were detected between pregnancy outcomes in patients who have been directly exposed to anti-TNF therapy during pregnancy and patients who had been previously exposed to this treatment. In other words direct exposure to anti-TNF agents during pregnancy was not associated with a higher frequency of adverse events in patients with inflammatory bowel disease. Argüelles-Arias et al.21 recently described a series of 12 pregnant women (4 with ulcerative colitis and 8 with Crohn disease) exposed to infliximab during pregnancy. Six of the patients received treatment throughout pregnancy, 2 decided to interrupt treatment, and in 3 cases, treatment was interrupted during the first trimester. There were 8 cesarean deliveries and 4 vaginal deliveries. None of the neonates had congenital anomalies, intrauterine growth restriction, or low birth weight, although there was 1 case of premature birth.
In the area of psoriasis and pregnancy, Puig et al.22 published the case of a pregnant woman with psoriasis of 20 years’ duration who developed generalized pustular psoriasis during her first pregnancy. The initial psoriasis was refractory to treatment with systemic corticosteroids and ciclosporin, and the patient was started on infliximab at a dose of 5mg/kg, which resulted in a 75% improvement in the Psoriasis Area Severity Index score (PASI 75) and complete clearance of lesions after 6 weeks of treatment. The patient became pregnant during treatment, which she continued for 19 months, with no detection of adverse effects or fetal anomalies. The patient became pregnant again and was exposed to infliximab during the first trimester. She gave birth to a healthy child.
In assessing the risk-benefit ratio for infliximab use during pregnancy, it is important to note that maternal exposure to infliximab can alter the normal maturation of the child's immune system, possibly leading to an increased risk of infection or abnormal response to vaccines.13 Vasiliauskas et al.23 described a case of maternal exposure to infliximab throughout pregnancy in which the child appeared to have a normal immune system at the age of 6 months, although low, yet therapeutic, levels of infliximab were detected in child's serum. The true extent of possible immunosuppression is, however, unknown and it would not be unreasonable to assume that detectable concentrations of neonatal serum infliximab could interfere with the child's capacity to fight tuberculosis or other infections and with tolerance of live attenuated vaccines. Mahadevan et al.24 did not observe any alterations in immune response to vaccinations in 8 children born to women exposed to infliximab throughout pregnancy. Nonetheless, a recent case report described a fatal case of disseminated tuberculosis infection in a child who had been born to a woman treated with infliximab throughout pregnancy. The child was given the Bacillus Calmette-Guérin vaccine at the age of 3 months and died a month and a half later. Most cases of disseminated tuberculosis have been described in immunocompromised patients, but in this case, it was not possible to evaluate the child's immune state or to determine the presence of immunodeficiency.25
The use of life attenuated vaccines is contraindicated in patients undergoing biologic therapy, and at the most recent World Congress of Gastroenterology, it was agreed to delay the administration of live attenuated vaccines in children exposed to biologic therapy in utero at least until circulating drug levels were no longer detectable (which may take several months).26 The long-term consequences of fetal exposure to biologic therapy on the immune systems of newborns are currently unknown.
AdalimumabAdalimumab is a recombinant human monoclonal antibody that binds with high specificity and affinity to TNF, thereby preventing it from binding to specific receptors.27 Most of the information on the use of adalimumab in pregnancy is from patients with rheumatoid arthritis and inflammatory bowel disease. In 2011, the OTIS published the results of a cohort study of 161 pregnant women (101 with Crohn disease and 60 with rheumatoid arthritis) exposed to adalimumab during the first trimester of pregnancy and compared them with results from a group of 68 pregnant women with rheumatoid arthritis, 11 with Crohn disease, and 134 healthy women not exposed to adalimumab.28 Of the women with rheumatoid arthritis treated with adalimumab, 3.4% had a child with a major congenital defect, compared with 4.6% of unexposed women with rheumatoid arthritis. The corresponding rates for Crohn disease were 14.1% and 10%, respectively. Major congenital defects were observed in 4.1% of children born in the healthy control group. The rates of spontaneous abortion in the groups exposed to adalimumab were 10% (rheumatoid arthritis) and 12.9% (Crohn disease), compared with 4.4% and 9.1% respectively in the unexposed groups and 1.5% in the group of healthy women. No statistically significant differences were found between the groups for the rate of congenital malformations, but there was a higher rate of spontaneous abortions in the adalimumab group. The authors suggested that the difference might be due to the fact that the women treated with adalimumab were enrolled in the study early on, when the risk of miscarriage is at its highest.
Dessinioti et al.29 recently described the case of a pregnant woman with psoriasis exposed to adalimumab during the first trimester who had a normal pregnancy and delivery and gave birth to a child with no evidence of birth defects.
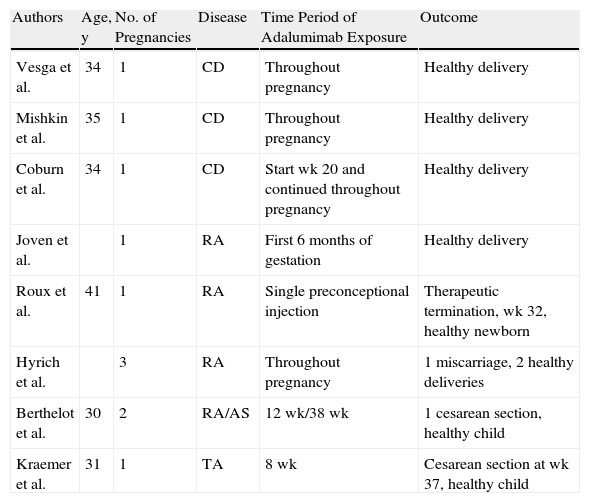
Table 1 shows the details of other isolated cases of exposure to adalimumab during pregnancy; based on these cases, there is no evidence of an increased risk of congenital malformations, abortions, or pregnancy-related complications.30
Isolated Cases With Evidence of Exposure to Adalimumab During Pregnancy.
| Authors | Age, y | No. of Pregnancies | Disease | Time Period of Adalumimab Exposure | Outcome |
| Vesga et al. | 34 | 1 | CD | Throughout pregnancy | Healthy delivery |
| Mishkin et al. | 35 | 1 | CD | Throughout pregnancy | Healthy delivery |
| Coburn et al. | 34 | 1 | CD | Start wk 20 and continued throughout pregnancy | Healthy delivery |
| Joven et al. | 1 | RA | First 6 months of gestation | Healthy delivery | |
| Roux et al. | 41 | 1 | RA | Single preconceptional injection | Therapeutic termination, wk 32, healthy newborn |
| Hyrich et al. | 3 | RA | Throughout pregnancy | 1 miscarriage, 2 healthy deliveries | |
| Berthelot et al. | 30 | 2 | RA/AS | 12 wk/38 wk | 1 cesarean section, healthy child |
| Kraemer et al. | 31 | 1 | TA | 8 wk | Cesarean section at wk 37, healthy child |
Source: Adapted from Jürgens et al.30
Abbreviations: AS, ankylosing spondylitis; CD, Crohn disease; RA, rheumatoid arthritis; TA, Takayasu arteritis.
As mentioned earlier, there is a scientific basis to investigate the therapeutic applications of TNF inhibitors in the area of what has been termed inflammatory infertility. Winger and colleagues have conducted several studies in which they attempted to demonstrate the effectiveness of anti-TNF therapy in women with inflammation-based fertility. In the first study,31 they compared the success of a combined regimen of anti-TNF therapy (adalimumab and etanercept) and intravenous immunoglobulin and oral anticoagulants versus oral anticoagulants alone in 75 patients with a history of recurrent spontaneous abortions and hereditary thrombophilia. The results were favorable, and the authors reported statistically significant differences in favor of the combined treatment group for the number of at-term pregnancies and low risk of pregnancy-related or perinatal complications. Another recent study published by the same authors32 evaluated the risk (in terms of birth defects) associated with the use of preconception adalimumab during in vitro fertilization (IVF) treatment in subfertile women with an elevated T helper 1/T helper 2 cytokine ratio. The group exposed to adalimumab received 2 doses separated by an interval of 2 weeks 41 to 65 days before embryo transfer. The birth defect rate was 2.44% in the exposed group and 2.11% in a similar group of women who did not receive the drug. These rates are comparable to those seen in the general IVF population. In the adalimumab group, there was 1 case of DiGeorge syndrome and in the non-adalimumab group there was 1 case of Edward syndrome. The authors did not observe an increase in birth defect rates in patients treated with preconception TNF inhibitor, but agreed that larger studies were needed to confirm this observation.
Because infliximab and adalimumab are IgG1 immunoglobulins, i.e., large hydrophilic molecules with an approximate molecular mass of 150kD, they can only pass through to the placenta by active receptor-mediated transport.3 The first step in maternal-fetal transfer of IgG takes place through a receptor known as the neonatal Fc receptor (FcRn). This is a heterodimeric molecule composed of an α chain and β2-microglobulin chain that binds to IgG with high affinity at a pH of 6 to 6.5. Transport capacity varies according to the subclass of IgG, based on the following sequence: IgG1>IgG4>IgG3>IgG2. Infliximab and adalimumab are both IgG1 antibodies. FcRn is strongly expressed in the syncytiotrophoblast during the first trimester of pregnancy, but not before week 14. Nonetheless, several authors have detected transport of IgG to embryo tissue at week 4 of gestation, suggesting the existence of an as yet unidentified alternative mechanism of immunoglobulin transport during the early weeks of pregnancy.3 Consequently, maternal-fetal transfer of infliximab and adalimumab would be minimal during the first trimester, but would subsequently increase over the course of the pregnancy due to the growth of the syncytiotrophoblast and the discontinuous layer of the cytotrophoblast, which would begin to form in the second trimester. FcRns have also been found on the surface of microvilli in the fetal intestine after 18 weeks of pregnancy. If we consider that the fetus starts to swallow amniotic fluid at the beginning of month 5 and that most amniotic protein fluid is cleared in this manner, fetal swallowing might constitute another mechanism of maternal-fetal transfer of infliximab and adalimumab.3
EtanerceptEtanercept is a fusion protein formed by the binding of the extracellular portion of the p75 receptor of TNF and the constant portion of human IgG1; it acts through competitive inhibition of TNF binding to cell surface receptors. Its efficacy has been demonstrated in the treatment of moderate and severe psoriasis in adults and children, psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, and juvenile idiopathic arthritis (rheumatoid arthritis).1
The British Society for Rheumatology identified 35 pregnancies in 11 437 women treated with anti-TNF drugs, and documented follow-up data in 32 of these. Twenty-nine patients had rheumatoid arthritis, 17 arthritis, 1 seronegative ankylosing spondylitis, and 1 juvenile idiopathic arthritis. Twenty-three of the women were being treated at the time of conception (17 with etanercept, 3 with infliximab, and 3 with adalimumab), and 11 women were also taking methotrexate and/or leflunomide. Just 2 patients continued with anti-TNF therapy during the first trimester; 1 continued up to week 20 and the other continued throughout pregnancy. There were 14 live births, 6 involuntary abortions (4 with etanercept, 1 with adalimumab, and 1 with infliximab; this group included the 3 patients taking concomitant methotrexate), and 3 elective abortions (3 with etanercept and 2 with methotrexate). There was no evidence of congenital anomalies or increased maternal-fetal toxicity. One of the patients with rheumatoid arthritis receiving etanercept and leflunomide at the time of conception gave birth to a healthy premature child at 34 weeks of gestation. Another patient, also with rheumatoid arthritis and being treated with etanercept at the time of conception, gave birth to a healthy child with a low birth weight (2180g). The patient who continued to receive etanercept during the second trimester underwent an emergency cesarean section due to intrapartum fetal distress. The patient treated with etanercept throughout pregnancy had a cesarean section at full term due to intrapartum bleeding. In this series of 32 women with rheumatic diseases exposed to anti-TNF agents at the time of conception or in the 10 previous months, there was no evidence of an increased incidence of congenital malformations, abortions, or complications during pregnancy compared with the general population.4
The OTIS analyzed follow-up data from 33 pregnant women with rheumatoid arthritis exposed to anti-TNF therapy during the first trimester of pregnancy (etanercept in 29 cases and infliximab in 4) between 1999 and 2004, and compared the results with those from 77 patients with rheumatoid arthritis who did not receive anti-TNF therapy and 50 healthy patients. In the etanercept group there were 3 spontaneous abortions and just 1 major congenital defect (trisomy 18). No statistically significant differences were detected for premature birth or low birth weight as compared with the control group of patients with rheumatoid arthritis who did not receive biologic therapy. There were, however, significant differences with the control group of healthy patients, leading the authors to suggest that the outcomes observed might be attributable to the underlying disease rather than to treatment with TNF inhibitors.33,34 In another retrospective study of 256 pregnant patients (102 with rheumatoid arthritis, 25 with psoriasis and psoriatic arthritis, 16 with ankylosing spondylitis, and 32 with other diseases) between 2005 and 2008, the OTIS compared pregnancy outcomes between women exposed to etanercept (n=175) and an unexposed control group (n=81). No statistically significant differences were observed for rate of abortions, premature births, or congenital malformations.34,35
Analysis of 3550 women with various rheumatic diseases treated with biologics in the Spanish BIOBADASER registry identified 14 pregnancies. Eight of the women had received etanercept, 4 infliximab, and 2 adalimumab. There were 7 births without complications (4 with etanercept and 3 with infliximab), 3 therapeutic terminations (2 with etanercept and 1 with adalimumab), and 1 spontaneous abortion (with infliximab).6
Strangfeld et al.36 recorded data from the German registry RABBIT, which collects data on patients with rheumatoid arthritis who start biologic therapy. They identified 37 pregnancies in 29 women who had been exposed to anti-TNF agents at the time of conception or at least during the first trimester. Two of the women had taken infliximab, 5 adalimumab, 20 etanercept, and 8 other types of anti-TNF agents. No statistically significant differences were observed for birth weight, and no congenital malformations were observed.
Viktil et al.37 recently published data from a series of 1461 pregnant women and 1198 fathers exposed to anti-TNF therapy 3 months before or at the time of conception. The drugs used were etanercept (n=37), adalimumab (n=3), azathioprine (n=101), methotrexate (n=8), leflunomide (n=2), sulfasalazine (n=119), hydroxychloroquine (n=58), prednisolone (n=633), and NSAIDs (n=723). None of the children born to mothers treated with adalimumab, etanercept, leflunomide, or methotrexate (prior to conception) developed malformations.
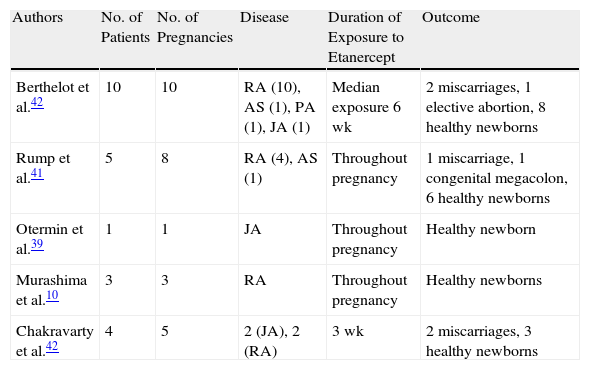
In 2011, the British Society for Rheumatology published data on 130 pregnancies in 118 women with rheumatoid arthritis exposed to anti-TNF agents before or during pregnancy.38 They evaluated and compared results according to 4 groups: exposure to anti-TNF therapy and methotrexate and/or leflunomide at the time of conception (group 1a, 21 pregnancies); exposure to anti-TNF agents at the time of conception (group 1b, 50 pregnancies); exposure to anti-TNF agents before conception (group 2, 59 pregnancies); and never exposure to anti-TNF agents (group 3, 10 pregnancies). The rate of spontaneous abortions was higher in the group exposed to anti-TNF therapy at the time of conception (33% in group 1a and 24% in group 1b) than in either group 2 (17%) or the control group (10%). Of the 88 live births in the anti-TNF groups, there were 11 premature births (26%) in group 1 and 8 (17%) in group 2. There were 4 fetal deaths in utero: 2 in group 1b and 2 in group 2. One child born to a mother who had been exposed to etanercept during the first trimester died 27 hours postpartum due to hypoxia, and there were 4 reports of congenital malformations: 2 in group 1b (congenital dislocation of the hip and pyloric stenosis) and 2 in group 2 (winking jaw syndrome and strawberry birth mark). Table 2 summarizes the pregnancy outcomes of other small cases series of women exposed to etanercept during pregnancy.10,39–42Several publications have suggested that etanercept, like adalimumab, might have beneficial effects in women with a history of infertility. Jerzak et al.,43 for instance, recently published a study investigating the effect of etanercept therapy on the activity of peripheral natural killer (NK) cells in 30 nonpregnant women with a history of recurrent miscarriage or failed IVF. They all received 4 doses of etanercept 25mg twice weekly before conception, and peripheral NK cell activity was evaluated by flow cytometry. The authors observed a decrease in NK cell activity in association with subsequent pregnancy. Murashima et al.10 published 3 cases of women with rheumatoid arthritis and a long history of infertility who became pregnant several months after starting treatment with etanercept to treat their arthritis.
Cases Series With Evidence of Etanercept Use During Pregnancy.
| Authors | No. of Patients | No. of Pregnancies | Disease | Duration of Exposure to Etanercept | Outcome |
| Berthelot et al.42 | 10 | 10 | RA (10), AS (1), PA (1), JA (1) | Median exposure 6 wk | 2 miscarriages, 1 elective abortion, 8 healthy newborns |
| Rump et al.41 | 5 | 8 | RA (4), AS (1) | Throughout pregnancy | 1 miscarriage, 1 congenital megacolon, 6 healthy newborns |
| Otermin et al.39 | 1 | 1 | JA | Throughout pregnancy | Healthy newborn |
| Murashima et al.10 | 3 | 3 | RA | Throughout pregnancy | Healthy newborns |
| Chakravarty et al.42 | 4 | 5 | 2 (JA), 2 (RA) | 3 wk | 2 miscarriages, 3 healthy newborns |
Abbreviations: AS, ankylosing spondylitis; JA, juvenile arthritis; PA, psoriatic arthritis; RA, rheumatoid arthritis; TA, Takayasu arteritis.
Although etanercept is not an immunoglobulin, it has a similar structure to immunoglobulins and therefore only minimal concentrations of the drug pass through to the placenta during the first trimester. This is not the case, however, during the second and third trimesters. Placental transfer of etanercept has been quantified through the measurement of etanercept concentrations in maternal serum, cord blood, and neonatal serum.44 It was found that cord blood concentrations were significantly lower than maternal serum concentrations during the second and third trimesters of pregnancy and that they decreased progressively in the child's serum after birth, indicating that etanercept placental transfer does occur but results in much lower concentrations than those seen in the mother.
UstekinumabUstekinumab is a human monoclonal antibody that binds with high affinity and specificity to the p40 subunit of interleukins 12 and 23, inhibiting their activity. There is very little experience with the use of ustekinumab to treat psoriasis due to its relatively recent appearance on the market, and to date, there have been no reports on its use in association with pregnancy. Martin et al.45 conducted a study in cynomolgus macaques to investigate the potential effects of ustekinumab treatment during pregnancy and breastfeeding. Possible effects on the immune system were evaluated by immunophenotyping of peripheral blood lymphocytes and immune histopathology of lymphoid tissues in macaque fetuses and infants and by T-dependent antibody response in infants. Treatment with ustekinumab did not produce maternal, fetal, or neonatal toxicity. The drug was detected in the serum of fetuses at day 100 of gestation and in the serum of infants 120 days postpartum. Low levels of the drug were observed in breast milk.
Breastfeeding and Biologic TherapyIt is not clear whether or not the use of anti-TNF agents during breastfeeding is safe. The immunoglobulin-like structure of infliximab and adalimumab could theoretically allow their passage into breast milk, but because they are long-chain protein molecules, they could also theoretically be inactivated by digestive enzymes in the gastrointestinal tract of the neonate.
Kane et al.46 reported the cases of 3 patients with Crohn disease who had been treated with infliximab up to week 30 of pregnancy and for the first 14 days postpartum; they found infliximab concentrations in maternal serum but not in breast milk or fetal serum. There have been other isolated reports that indicate that infliximab might be safe during breastfeeding, as no evidence of neonatal toxicity was found during its use.
Like infliximab, adalimumab could also be transferred through breast milk, but there is no scientific evidence from animal or human studies and it is therefore impossible to make recommendations.
The few studies that have been published on etanercept would appear to support the relative safety of its use during breastfeeding, as minimal concentrations of the drug have been detected in breast milk, with no or almost no neonatal absorption. Ostensen et al.47 described the case of a 30-year-old pregnant woman with rheumatoid arthritis treated with etanercept 25 mg twice weekly. Treatment was recommenced 4 weeks postpartum due to acute flares, and low concentrations of the drug were found in breast milk samples. Murashima et al.48 published the case of a 40-year-old pregnant women with rheumatoid arthritis treated with etanercept 25mg twice weekly; treatment was discontinued after delivery. Etanercept concentrations in the infant's serum decreased progressively and were undetectable by week 12 postpartum. Minimal concentrations were still, however, detectable in the mother's milk, suggesting that the drug might be transmitted through the placenta but not breast milk. Berthelsen et al.49 described the case of a 34-year-old patient with ankylosing spondylitis treated with etanercept 25mg twice weekly throughout pregnancy and breastfeeding. Very low drug concentrations were detected in breast milk and the levels present in the infant's serum decreased rapidly, supporting the theory that the drug is not passed through breast milk.
The little experience accumulated to date with the use of biologic agents during breastfeeding suggests an adequate safety profile, although there are data indicating that low drug concentrations may be present in breast milk, and it is not known how exposure to anti-TNF agents during breastfeeding could affect the still immature immune system of the child in the medium and long term.
ConclusionsThere is very little information on the use of biologic therapy during pregnancy and the information that is available is based on retrospective, observational studies, as for ethical reasons, clinical trials investigating the safety of biologics cannot be performed in pregnant women. Although studies have been conducted in animals, results cannot always be used to reliably predict responses in humans, and they do not guarantee safety because of interspecies differences due to, among other factors, differences in drug metabolization.
Most of the data published are based on series of patients with inflammatory arthritis and inflammatory bowel disease, and experience with the use of biologic agents in psoriasis is still very limited. The inclusion of certain study variables can also make it difficult to interpret data. With the exception of some specific studies, there have been no systematic attempts to correlate the underlying disease with the course of pregnancy in patients exposed to biologic therapy, and furthermore, some patients are also taking other drugs at the time of conception.
Few adverse effects related to the use of biologic therapy during pregnancy have been reported (e.g., premature birth, miscarriage, low birth weight, and preeclampsia), and the rates are similar to those seen in the general unexposed population. There have also been few cases of fetal malformations and congenital anomalies, and again, their incidence appears to be similar to that observed in the general population, and additionally, the association with biologic therapy has not been demonstrated. Most women with psoriasis or another immune-mediated inflammatory disease interrupt biologic therapy on discovering they are pregnant, and relatively few cases of women who continue to take the drug throughout pregnancy have been reported.
To establish the risk associated with exposure to biologic therapy at the time of conception and during pregnancy, all cases of pregnancy in women treated with anti-TNF agents and ustekinumab should be documented, and long-term follow-up should be conducted to further investigate the link between biologic therapy and the occurrence of congenital anomalies.
The data in the literature appear to indicate that biologic therapy during pregnancy has an adequate safety profile. However, there is no absolute evidence. Consequently, the decision to use biologics during pregnancy should be adequately evaluated on a case-by-case basis, with careful weighing up of the importance of maintaining adequate disease control and the potential risks of fetal damage.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Ruiz V, Manubens E, Puig L. Psoriasis y embarazo: revisión (II). Actas Dermosifiliogr. 2014;105:813–821.