Contact dermatitis due to cosmetic products is a common dermatologic complaint that considerably affects the patient's quality of life. Diagnosis, treatment, and preventive strategies represent a substantial cost. This condition accounts for 2% to 4% of all visits to the dermatologist, and approximately 60% of cases are allergic in origin. Most cases are caused by skin hygiene and moisturizing products, followed by cosmetic hair and nail products. Fragrances are the most common cause of allergy to cosmetics, followed by preservatives and hair dyes; however, all components, including natural ingredients, should be considered potential sensitizers. We provide relevant information on the most frequent allergens in cosmetic products, namely, fragrances, preservatives, antioxidants, excipients, surfactants, humectants, emulsifiers, natural ingredients, hair dyes, sunscreens, and nail cosmetics.
La dermatitis de contacto por cosméticos es un problema dermatológico frecuente, creciente, con un gran impacto en la calidad de vida de los pacientes que lo padecen y con un importante coste invertido en la búsqueda de estrategias diagnósticas, terapéuticas y de prevención. Su prevalencia se ha estimado entre el 2 y el 4% de las consultas dermatológicas, y aproximadamente el 60% de los casos son de causa alérgica. Los productos de higiene e hidratación cutánea son los responsables de la mayoría de los casos, seguidos de los cosméticos ungueales y capilares. Las fragancias son la causa más frecuente de alergia a cosméticos, seguidos de los conservantes y los tintes capilares; pero todos los componentes, incluyendo los ingredientes naturales, deben ser considerados como potenciales sensibilizantes. A lo largo de este trabajo se detallarán los datos relevantes de los alérgenos más frecuentes de los productos cosméticos: fragancias, conservantes, antioxidantes, excipientes, surfactantes, humectantes y emulsificantes, ingredientes naturales, tintes capilares, fotoprotectores y cosméticos ungueales.
European legislation defines a cosmetic as a substance or mixture of substances for application to external surfaces of the human body (epidermis, hair, lips, and external genitalia), teeth, or mucosa of the oral cavity with the only or principle aim of cleaning, perfuming, or modifying its appearance, and/or masking body odors. Personal hygiene products (e.g. gels and soaps) and moisturizers (e.g. creams and lotions), hair care products (e.g. shampoos and hair dyes), toothpaste, make-up, nail products (e.g. nail polish and artificial nails), fragrances (e.g. deodorants and perfumes), hair removal products, and sunscreens are all included within this definition. These cosmetics can be classified as stay-on or leave-on, that is, they remain in contact with the skin surface, or rinse-off or wash-off, that is, they are removed with water after a few minutes.1
EpidemiologyCosmetic contact dermatitis accounts for between 2% and 4% of visits to the dermatologist,2 although this figure probably underestimates the true prevalence because most of the patients with mild contact eczema do not seek specialist attention and simply avoid the cosmetic suspected to be responsible.3,4 Approximately 17% of patients who undergo patch testing in a skin allergy unit have lesions consistent with potential sensitization to cosmetics, with 59.04% testing positive in at least one test, and with a higher prevalence among women than men.5 Skin hygiene products and moisturizers are the cause of most cases of contact dermatitis, followed by make-up, and hair and nail products.6–8
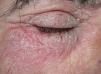
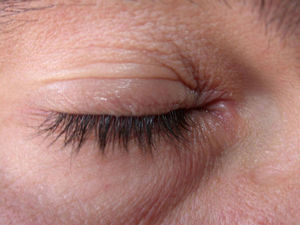
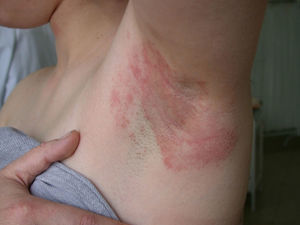
Clinical CharacteristicsThe clinical manifestations of cosmetic contact dermatitis depend on several factors such as the product used, the site of application, frequency of use, duration of contact, and certain individual patient characteristics.9,10 The facial region is the most frequent site for contact dermatitis, with the eyelids being particularly affected (Figs. 1 and 2).9
Main AllergensIdentification of the allergens responsible for cosmetic allergic contact dermatitis (ACD) took on particular importance from 1997 onwards, when the chemical composition had to be included by law on the label for cosmetics.2
Fragrances are the most common cause of allergies to cosmetics,5,7,9–12 followed by preservatives and hair dyes.2,5,7–10 Approximately half the positive tests are detected using a standard test battery,2,13 and of the allergens included in these batteries, Kathon CG (methylchloroisothiazolinone/isothiazolinone) and paraphenylenediamine (PPD) are the most prevalent.2,5
When a cosmetic is suspected of being responsible for contact dermatitis, and with a view to improving diagnostic yield, it is important to extend the battery with products used by the patient, as it is estimated that 15% of patients test positive to at least one of their own cosmetics.14
Below, we will detail the most relevant information on the most common allergens. First, we will present the allergens found in many cosmetics (fragrances, preservatives, antioxidants, excipients, emulsifiers, wetting agents, surfactants, and natural ingredients) and then those specific to certain categories (hair dyes, sunscreens, and nail cosmetics).
FragrancesA fragrance is any basic ingredient used in the production or manufacture of materials because of their smell. A perfume is a creative composition or composite product of between 10 and 300 fragrances.12
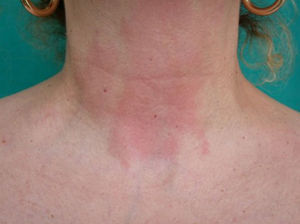
Fragrances are the second most frequent cause of contact allergy in Spain after metals,15–18 with positive results in 1.7% to 15.1% of patch tests.19–21 They are the most frequent cause of cosmetic allergies (Fig. 3).9,10,12,22,23
Since 2005, current European regulations require 26 fragrancies (Table 1) known to be contact allergens to be disclosed on the label of cosmetic and domestic products if their concentrations exceed 10 parts per million (ppm) in leave-on products and 100ppm in rinse-off products.12 It is estimated that the prevalence of sensitization to these products is 7.6%.19
List of 26 Fragrances That Must be Included on Label of Cosmetic Products, According to the Seventh Amendment of the European Cosmetics Directive.
| Cinnamal | Farnesol |
| Geraniol | Benzyl alcohol |
| Evernia prunasti | Benzyl salicylate |
| Cinnamyl alcohol | Linalool |
| Eugenol | Limonene |
| Hydroxycitronellal | Butylphenyl methylpropional |
| Isoeugenol | Anisyl alcohol |
| α-Amylcinnamal | Benzyl cinnamate |
| Citral | Benzyl benzoate |
| Coumarin | Methyl 2-octionate |
| Hydroxyisohexyl 3-cyclohexane carboaldehyde | Evernia furfuracea |
| α-Isomethyl ionone | Amylcinnamaldehyde |
| Citronnel | α-Hexylcinnamal |
The cosmetics most closely associated with sensitization to fragrances are perfumes, with leave-on products being more often responsible for allergy to fragrances than rinse-off products.24 Hydroxycitronellal and geraniol are the fragrances most widely found in perfumes, whereas linalool, limonene, and citronellol are the ones most widely found in hygiene products and daily moisturizers.12 An important allergen in ACD to deodorants is hydroxyisohexyl 3-cyclohexene carboxaldehyde, known as Lyral25 (Fig. 4).
Since January 1, 2012, the Spanish Contact Dermatitis and Skin Allergy Research Group (GEIDAC) has included fragrance mix ii in the standard test battery. This has led to a 15% increase in the sensitivity for detection with respect to traditional markers for fragrances, such as balsam of Peru and fragrance mix i.20,26
Balsam of Peru is a natural resin extracted from the Myroxylon pereirae tree. It includes approximately 250 different chemical substances, many of them unidentified. Some of them are fragrances such as cinnamyl alcohol, cinnamic aldehyde, eugenol, and isoeugenol. As a marker of fragrances, it tests positive in approximately 50% of patients with suspected fragrance allergy.23,26
Fragrance mix i contains 1% of each of the following 8 substances: amylcinnamaldehyde, cinnamyl alcohol, cinnamic aldehyde, hydroxycitronellal, geraniol, eugenol, isoeugenol, and oak moss extract or Evernia prunaastri,26 this latter substance being the most common allergen and amylcinnamaldehyde the least frequent in this group.21 Some fragrance mix i batteries contain 5% sorbitan sesquioleate to improve fragrance dispersion. Given that this substance is, in itself, allergenic, it is estimated that approximately 17.7% of the positive results for fragrance mix i are in actual fact false positives resulting from sensitization to this emulsifier. It is therefore recommended to include sorbitan sesquioleate in the specific fragrance battery.21,26 The estimated prevalence of sensitization to fragrance mix i in the general population ranges from 7.2% to 11.5%.27 Fragrance mix i is the most relevant for diagnosis of allergies to fragrancies,28 and can identify between approximately 70% and 80% of cases.26
Fragrance mix ii includes 6 fragrances, which in order of decreasing prevalence are Lyral, 3,7 dimethyl-[2,6]-octadienal or citral, farnesol, hexyl cinnamic aldehyde, coumarin, and citronellol.26,29 The prevalence of positive reactions in the population is estimated to be 5%, and it is considered the second most important marker for detecting allergies to fragrances. If this mix were not included in the standard battery, approximately 15% of patients allergic to fragrances would not be identified.28–30 Overall, 42% of patients who react positively to fragrance mix ii also have a positive reaction to fragrance mix i.29
Lyral is a very common allergen in Europe, with an estimated prevalence in the general population of 1% to 3%.26 In 2003, legislation limited the maximum concentration in both leave-on and rinse-off cosmetics to 1.5%.31 Since then, a decrease in the sensitization to this product has been observed.32 For individuals already sensitized to this compound, a concentration in cosmetics in the range of 0.009% to 0.027% is recommended.33
Whenever one or several of the fragrance markers in the standard battery test positive, it is recommended to test using the specific battery and complete the study by testing with some of the patient's own products.19,20 It has been shown that allergenicity is higher when fragrance mixes are used rather than each fragrance in isolation34 and that irritated skin is a risk factor for developing polysensitization to fragrances.35
In addition, some emerging fragrances have recently shown significant sensitization rates. One of these is linalool (dimethyl octadienol), a fragrance present in natural form in many essential oils. Rates of sensitization have been estimated at 1.3%.26 As a pure substance, its potency for sensitization is low, but its oxidation products are highly allergenic.36,37 Another fragrance not included either in one of the mixes or in the specific battery, with a prevalence of sensitization of 0.5%, is majantol (trimethyl-benzenepropanol).26
PreservativesTo prevent biological degradation of cosmetics by contaminating microorganisms, preservatives are added to the composition.38 There are a wide variety of such products on the market. However, the ideal preservative, a stable, nontoxic and nonirritant product with a broad antimicrobial spectrum, effective over a large pH range, and without any sensitizing capacity has yet to be discovered.39 Parabens are the most widely used such agents in cosmetics, followed by formaldehyde-releasing agents, and isothiazolinones.10,40 The preservatives with highest prevalence of sensitization are methyldibromo glutaronitrile, formaldehyde, and Kathon CG, while the parabens are the agents with lowest prevalence.41
ParabensParabens are a family of akyl esters (methyl-, ethyl-, propyl-, butyl-, and benzylparaben) of p-hydrobenzoic acid. In view of their optimal properties, these substances are one of the most widely used preservatives in cosmetics, drugs, and food.9 In fact, they are the most widely used preservative in cosmetic products39 and the United States Food and Drug Administration ranks them second in the most widely used ingredients in cosmetic formulations, surpassed only by water.42 In a European study, 99% of leave-on cosmetics and 77% of rinse-off ones had parabens in their composition.10
Their capacity to act as sensitizers when used in cosmetics (applied to healthy skin) is low, at around 1%, and in fact they have one of the lowest rates of sensitization of all preservatives.9,41,42 However, cases of sensitization with therapeutic preparations (applied to damaged skin) are much more numerous. It is surprising to observe that often individuals who have developed ACD to parabens after application to damaged skin can use paraben-containing cosmetics and that patients sensitized topically can fully tolerate oral intake of parabens. The contradictory behavior of these substances has been denoted the paraben paradox.42
Formaldehyde and Formaldehyde-Releasing AgentsFormaldehyde is an extremely ubiquitous allergen, as in addition to being used specifically as a preservative in many cosmetics and domestic and industrial products, it is also present as a contaminant in many other products.43 It is a frequent cause of ACD, with an estimated prevalence of sensitization of 2% in Spain43,44 and of 8% to 9% the United States.45 De Groot46 showed that there are similar percentages of formaldehyde-containing cosmetics in the United States and Europe, and so the different sensitization rates could be explained by differences in legislation. In the European Union, the maximum formaldehyde concentration is limited to 0.2% (0.1% in oral hygiene products) whereas the United States does not have any specific regulations.43,45 Although their use, and hence also the prevalence of sensitization, has decreased through replacement with safer preservatives such as formaldehyde-releasing agents,9,47 an increase has once again been detected.23,41
Formaldehyde-releasing agents are molecules that, in the presence of water, release formaldehyde in varying quantities depending on the type of preservative used, its concentration, and the amount of water present in the product.39 Although more than 40 substances have been described, a skin allergy unit will typically have to deal with only a few of these. The most widely used agents in cosmetic products, in order of increasing extent of formaldehyde release, are as follows: 2-bromo-2-nitropropane-1,3-diol (bronopol)<imidazolidinyl urea<dimethylol-dimethyl hydantoin (DMDMH)<diazolidinyl urea<quaternium 15.43 As with formaldehyde, the prevalence of sensitization to formaldehyde-releasing agents is higher in the United States than in Europe.43,45,48
Approximately 34% of patients allergic to formaldehyde are also sensitized to a formaldehyde-releasing agent, in particular quaternium 15 and, to a lesser extent, bronopol.44 It has been estimated that 15% of positive reactions to bronopol and 40% to 60% of positive reactions to other formaldehyde-releasing agents are due to formaldehyde release.49 However, different studies have shown that not all allergies to formaldehyde-releasing agents are due to formaldehyde release, but rather there are other components of these substances that can act as allergens and induce sensitization.44,50
Quaternium 15In Europe, the prevalence of sensitization to quaternium 15 ranges from 0.6% to 1.9%,51 and is estimated to be 1.27% in Spain.43 It is the molecule of this class of preservatives that releases most formaldehyde and the one with the greatest sensitizing capacity.39,43,47 More than half the patients who react positively to quaternium 15 also react to formaldehyde and approximately 40% of patients sensitized to formaldehyde are also sensitized to quaternium 15.52 This molecule is the only formaldehyde-releasing agent included in the standard battery, although some centers also include diazolidinyl urea and imidazolidinyl urea in their test batteries in an effort to improve diagnosis.48
Diazolidinyl UreaCompared with imidazolidinyl urea, diazolidinyl urea releases more formaldehyde, has a broader antimicrobial spectrum and a stronger sensitizing capacity, and is a better preservative.39 In Europe, the prevalence of sensitization to diazolidinyl urea ranges from 0.5% to 1.4%, with 24% to 74% of positive reactions considered relevant.43,48 Patients allergic to diazolidinyl urea show cross-reactivity to formaldehyde and other formaldehyde-releasing agents.47 Decomposition of the molecule yields at least 4 products, of which (4-hydroxymethyl-2,5-dioxoimidazolidine-4-yl)-urea (HU) and (3,4-bis-hydroxymethyl-2,5-dioxoimidazolidine-4-yl)-urea (3,4-BHU) are the most relevant in cosmetics.53 Both HU and 3,4-BHU are also present as products of the degradation of imidazolidinyl urea. This might explain the large number of cases of formaldehyde-independent cosensitization reported for these 2 agents.43,54,55 Between 12% and 81% of patients with sensitization to diazolidinyl urea also react to formaldehyde.48
Imidazolidinyl UreaImidazolidinyl urea is, after bronopol, the preservative in this class that releases least formaldehyde,43 approximately an eighth the quantity released by quaternium 15.39 In Europe, the prevalence of sensitization to imidazolidinyl urea ranges from 0.3% to 1.4%, with 21% to 90% of positive reactions considered relevant.43,48 Imidazolidinyl urea is formed of at least 7 chemical compounds, with allantoin, HU, 3,4-BHU and (3-hydroxymethyl-2,5-dioxo-imidazolidinyl-4-yl)-urea being the main degradation products.55 Imidazolidinyl urea shows cross-reactivity to both formaldehyde and other formaldehyde-releasing agents.47 Cross-reactivity to diazolidinyl urea is common. Degradation products shared by both molecules include HU and 3,4-BHU, which are among the molecules responsible for cosensitization.55 Approximately 11% and 63% of patients who react to imidazolidinyl urea also show reactivity to formaldehyde.43 When imidazolidinyl urea or diazolidinyl urea show cross-reactivity to quaternium 15, the responsible molecule is considered to be formaldehyde, as the agents are not structurally related.43
Dimethylol Dimethyl HydantoinNo current data are available on the prevalence of sensitization to dimethylol dimethyl hydantoin (DMDMH) in Europe. In the United States, the prevalence is estimated between 0.5% and 3.4%, and 15% to 86% of positive reactions are considered relevant.48 DMDMH, imidazolidinyl urea, and diazolidinyl urea belong to the group of hydantoins, and this could explain the cases of formaldehyde-independent cosensitization to these chemically related molecules.43,50 Approximately 1 in 5 patients sensitized to formaldehyde shows reactivity to DMDMH.43
BronopolThe prevalence of sensitization to bronopol in Europe is lower, with estimates ranging from 0.4% to 1.2% and between 7% and 80% of positive reactions being relevant.48 Decomposition of bronopol yields formaldehyde, bromonitroethanol, and tris-(hydroxymethyl)nitromethane.50 In fewer than 25% of cases of sensitization to bronopol, patients also react to formaldehyde, while fewer than 10% of patients allergic to formaldehyde react to bronopol.43 In addition, sensitivity to this preservative is not usually associated with other formaldehyde-releasing agents.43
OthersOther formaldehyde-releasing preservatives used in cosmetic products include benzyl hemiformal, 5-bromo-5-nitro-1,3-dioxane, and sodium hydroxymethylglycinate, but these agents are rarely sensitizers or, at least, there is little published to suggest that they can have such an effect.48
Currently, there are no published studies with sufficient data to determine whether formaldehyde-releasing agents are really a risk for patients sensitized to formaldehyde. In the literature, there is still certain debate about the therapeutic approach in patients allergic to formaldehyde. Although some authors suggest that it is not necessary to avoid all formaldehyde-releasing agents,56 most recent articles recommend patients allergic to formaldehyde to also avoid formaldehyde-releasing products, and in the event that no alternative is available, it is recommended to use products with bronopol as a preservative after performing a patch test.43,49
IsothiazolinonesIsothiazolinones are among the most frequently used preservatives; it is estimated that 23% of preservatives contain isothiazolinones.40
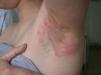
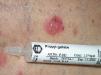
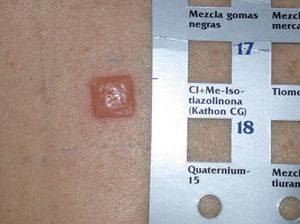
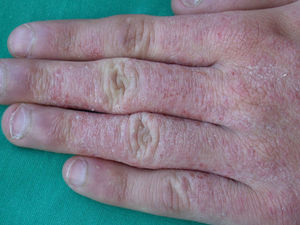
Kathon CG is a mixture of methylchloroisothiazolinone (MCI) and methylisothiazolinone (MI) in a 3:1 ratio, with MCI being the most allergenic component of the combination.57,58 Along with formaldehyde, quaternium 15, iodopropynyl butylcarbamate, and methyldibromo glutaronitrile (MDBGN), this agent is one of the most frequent causes of contact allergy to preservatives; however, of these 5 agents, MCI/MI is the most clinically relevant.59 In view of its strong sensitizing capacity, the maximum concentration allowed in Europe was set to 15ppm in both rinse-off and leave-on cosmetics (in the United States, the concentrations allowed are 15ppm in rinse-off products and 7.5ppm in leave-on cosmetics).60 Despite this change in legislation, an increase in the prevalence of sensitization to this preservative has been reported, from 2.3% in 2009 to 3.9% in 2011.61 It has been suggested that the increased use of MI without MCI as a preservative has led to greater sensitization to MI, and as a result of cross-reactivity, to an increase in positive reactions to MCI/MI.61 Although this agent is included in the standard battery at a concentration of 100ppm aq., it is estimated that between 24% and 50% of cases are not diagnosed at this concentration60 (Figs. 5 and 6).
MI was selected as the contact allergen of the year in 2013 by the American Society of Contact Dermatitis.59 As a preservative, it is less effective than Kathon CG, and so higher concentrations are required (100ppm) but it also has a lower sensitizing capacity.62 The prevalence of sensitization to MI in Europe is estimated to be 1.5%,59 although between 2009 and 2011, there was a substantial increase in sensitization to this preservative.61 The percentage of cosensitizations to both compounds is variable, with between 40% and 67% of patients who react to MI also reacting to MCI/MI.59 If sensitization to isothiazolinones is suspected, both the MCI/MI mixture and MI alone should be included in the patch test, because if only MCI/MI is included, approximately 40% of allergies to MI are not diagnosed. This is because the concentration of MI in the Kathon CG patch (25ppm) is much lower than in the patch with the preservative by itself (75ppm).59,62
Methyldibromo GlutaronitrileMDBGN was introduced into Europe in 1985. It is either used by itself or in combination with phenoxyethanol (1:4), in which case it is known as Euxyl K400, with MDBGN being the main source of sensitization (phenoxyethanol is rarely the cause of contact allergy).63,64 The incidence of sensitization to this preservative increased progressively until reaching a prevalence in Europe of approximately 4% in 2005.41 Since then, regulatory measures have been taken, culminating in 2008 with the prohibition of the use of MDBGN in leave-on cosmetics. As a result, there has been a significant decrease in contact allergies to this preservative.65 A concentration of 50ppm has been found to be safe for sensitization to MDBGN.66
Iodopropynyl Butyl CarbamateIodopropynyl butyl carbamate has been introduced recently as a preservative in cosmetic products, in particular, in sanitary towels.67,68 It is one of the most frequent causes of contact allergy to preservatives.59
OthersThere are many other preservatives used in cosmetic products, although they are of considerably less importance in terms of frequency of use and/or sensitizing capacity. Examples include thimerosal, triclosan, sorbic acid, benzalkonium chloride, chloroacetamide, captan, cetyl alcohol and stearyl alcohol,11 isopropyl alcohol,69 and sodium dehydroacetate.70
AntioxidantsAntioxidants are molecules that protect other molecules against the effect of free radicals, avoiding or delaying oxidation, and so preventing the product from acquiring a stale appearance or a disagreeable smell. The molecules are considered a minority group within cosmetic allergens. The main sensitizers in this group include galates, followed by others such as butyl hydroxyanisole, butylhydroxytoluene, ter-butylhydroquinone, and nordihydroguaiaretic acid.10,57 Other antioxidants, which are used mainly in sunscreens and antiaging creams, and include tocopheryl acetate (vitamin E), retinyl palmitate, and ascorbic acid (vitamin C) are rarely responsible for ACD.23 However, there have been recent cases of ACD from hydroxydecyl ubiquinone or idebenone (synthetic analogue of coenzyme Q10).71–73
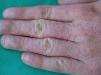
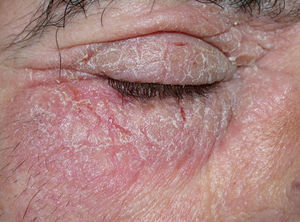
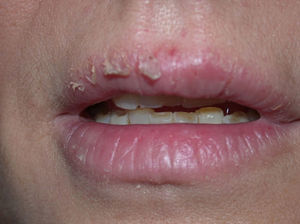
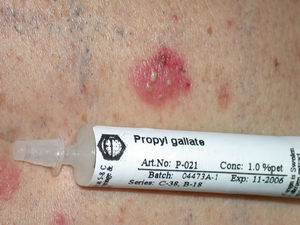
GalatesGalates are alkyl esters of trihydroxybenzoic acid, with propyl galate, octyl galate, and dodecyl galate being the most widely used. Although these allergens are ubiquitous, cases of sensitization are relatively rare, with the prevalence estimated to be 3.92%, possibly because oral tolerance develops through repeated exposure.,25,74 Propyl galate is responsible for the most cases of ACD because, although its sensitizing capacity is low, it is the most widely used.75 Cosmetics, and specifically lipsticks, are the products most frequently involved in cases of sensitization to these molecules. This explains why cheilitis is the most common clinical manifestation (Figs. 7 and 8).25,74,75
Excipients, Surfactants, Wetting Agents, Emulsifiers, and OthersSubstances such as excipients, surfactants, wetting agents, and emulsifiers are common ingredients in cosmetic products, but they are not considered a frequent cause of skin allergies to cosmetics. Of particular note are traditional substances such as propylene glycol, fatty alcohols such as cetyl alcohol, and wool alcohols.23 However, over time, new molecules have come onto the market and with them publications about the allergenicity of these new agents in cosmetics. Some of the more recently identified compounds include dicaprylyl maleate,23,67 cocamidopropyl betaine (CAPB),23,64,76,77 ethylhexylglycerin,23,78 bis-diglyceryl polyacyladipate-2,79,80 octyldodecyl xyloside,81 pentylene glycol,23,82 isononyl isononanoate and trioleyl phosphate,23,83 kerosene84 dithiocarbamates,85 tetrahydroxypropyl ethylenediamine,86 and copolymers such as C30-38 olefin/isopropyl maleate/maleic anhydride.87
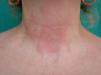
Cocamidopropyl BetaineLeave-on cosmetics have been considered as infrequent causes of ACD because, apart from the short time of contact with the skin before rinsing, the allergens contained by these products usually have little sensitizing capacity. An exception is CAPB, a nonionic surfactant with limited irritative capacity that is manufactured by a chemical reaction between fatty acids extracted from coconut oil and 3-dimethylaminopropylamine, the latter compound being the main allergenic fraction.9,64,76,77 This product has been a relatively frequent cause of sensitization in ACD to shampoos (Fig. 2); however, the current prevalence of sensitization is low (0.27%).10
Natural IngredientsNatural ingredients derived from plants (for example, almond, wheat, soy, and sesame) are increasingly added to cosmetics, mainly as fragrances, but also taking advantage of their antiinflammatory, antioxidant, and antipruritic properties.88,89 Among the patients with suspicion of ACD to cosmetics, there is a high prevalence of sensitization to plant extracts, with the most frequent being allergy to tea tree oil90 and derivatives of the Compositae and asteraceas family.91
The main problem is that these ingredients are not correctly labelled (for example, the essential oils are not recognized as fragrances) and so it is hard for the patient to identify them as potential allergens. It is therefore recommended that patients allergic to fragrances also avoid contact with cosmetics that include plant extracts in their composition.23,90
Hair Dyes and Hair ProductsAlthough most of the cases described in the literature of ACD to hair dyes are due to p-phenylenediamine (PPD), many other substances present in hair dyes could be considered as agents with a high sensitizing capacity.92
p-PhenylenediaminePPD is a product that is used as an ingredient in permanent and semi-permanent hair dyes.93 Although this ingredient is also used as a dye in other cosmetic and noncosmetic products,10 most sensitizations to PPD occur due to contact with hair dyes; indeed there is a significant association between positive reaction to PPD and being a hairdresser.93 The agent is a frequent cause of ACD, with an estimated prevalence of sensitization to PPD in Europe of 4%, and a relevance of positive reactions ranging from 44% to 64%.94
The PPD derivatives, p-amino diphenylamine, o-nitro-p-phenylenediamine, and p-toluenediamine, are a less frequent cause of skin allergy to hair dyes, and sensitization may be explained by cross-reactions between these substances.93
Other SensitizersOther ingredients present in hair dyes, such as resorcinol, m-aminophenol, and 4-amino-2-hydroxytoluene, may be a cause of ACD.92,95 In addition to sensitization through contact at the hairdressers, bleaches (persulfate salts),96 permanent products (glyceryl thioglycolate), preservatives, perfumes, surfactants in shampoos and conditioners (CAPB, hydrolyzed animal proteins) may also be responsible for allergies.10,23,97
Nail Cosmetics: Nail Varnishes and Artificial NailsNail varnishes are composed of several types of ingredient: resins, solvents, plasticizers agents, dyes, thixotropic agents, and color stabilizers. Most allergic reactions to nail varnishes are caused by toluenesulfonamide formaldehyde resin, with an estimated prevalence of 4%. It is important to remember that more than 80% of cases of ACD appear in areas distant to the contact area (lips, eyelids, neck, and other areas of the face). Some of the other ingredients in nail varnishes, such as formaldehyde, nitrocellulose, and other resins, among others, may also be a cause of ACD although much less frequently.9,10,23,39,64,98
There are several types of artificial nails on the market: acrylic, porcelain, and gel. The main agents responsible for ACD caused by these products are acrylates. In particular, 3- metaacrylates are the most frequent allergens, namely, ethylene glycol dimethacrylate, 2-hydroxyethyl methacrylate, and 2-hydroxypropyl methacrylate. For most patients, the clinical presentation is one of eczema on the fingertips and/or the nail folds, at times accompanied by variable degrees of nail dystrophy, but distant lesions can also appear at sites such as the face (eyelids) and neck.98–101
SunscreensThe use of photoprotective agents, whether sunscreens per se or in cosmetics, has increased greatly in recent years due to recognition of the carcinogenic and skin aging effects of UV radiation. The most frequent adverse reaction to the use of these substances is irritation, which occurs in more than 15% of users.64 The prevalence of sensitization among patients with suspected ACD is low, probably less than 1%.10 Benzophenones—and oxybenzone and dibenzoylmethanes (avobenzone) in particular—are the main sensitizing agents in sunscreens. p-Aminobenzoic acid and its derivatives are not widely used today, and so their prevalence as allergens is low. To date, there have been no reports of allergy caused by physical filters such as zinc oxide and titanium dioxide.102,103 In addition to the substances used as sunscreens, other ingredients may be the cause of ACD.104
ConclusionContact dermatitis to cosmetics is a common and growing problem in dermatology. As such, dermatologists should recognize the problem and be familiar with it.
Fragrances and preservatives are the most frequent allergens in cosmetics; however, all ingredients should be considered as potential sensitizers.
Faced with clinical suspicion of ACD due to cosmetics, standard and specific patch testing, along with testing with the products used by the patients, should be carried out. Once the responsible allergen has been identified, we should inform and guide the patient about which products to use and which to avoid.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Dermatitis alérgica de contacto a cosméticos. Actas Dermosifiliogr. 2014;105:822–832.