Psoriasis is a complex inflammatory disease, and in women the incidence is high in child-bearing years. Treatment during pregnancy presents genuine challenges since management requires adequate assessment of the extent of disease, comorbidity, and potential risk to the fetus. Scientific evidence is scarce on the effects that certain drugs have on fetal development given the ethical concerns about enrolling pregnant women in clinical trials. This review presents up-to-date information on the course of psoriasis during gestation and discusses associated conditions and the therapeutic protocols recommended for use during pregnancy.
La psoriasis es una enfermedad inflamatoria compleja que, en el caso de las mujeres, tiene una alta incidencia durante su etapa reproductiva. El tratamiento de la psoriasis durante el embarazo representa un verdadero reto terapéutico ya que hemos de valorar adecuadamente la extensión de la enfermedad, las comorbilidades asociadas y el potencial riesgo fetal. Actualmente disponemos de escasa evidencia científica que defina el efecto exacto del uso de determinados fármacos sobre el desarrollo embrionario o fetal en gestantes con psoriasis debido a la ausencia de ensayos clínicos llevados a cabo en esta población por razones éticas. Esta revisión intenta proporcionar datos actuales relativos a curso de la psoriasis durante la gestación, comorbilidades asociadas y pautas terapéuticas recomendadas en este contexto.
Psoriasis is a chronic, complex, multifactorial inflammatory disease that affects both sexes equally. The mean age of onset in women is around 28 years, and 75% of female patients develop psoriasis before they are 40 years old, meaning that the majority of women are in their childbearing years at the time of diagnosis.1 The fact that psoriasis is associated with comorbid conditions, such as diabetes, metabolic syndrome, and cardiovascular disease, and that several studies have linked psoriasis in pregnancy to certain modifiable risk factors, such as overweight, smoking, depression, and maintenance of systemic inflammation, gestational, perinatal, and neonatal complications are a possibility in pregnant women with psoriasis.2 Understanding the above factors is crucial for the adequate management of psoriasis in pregnancy and for ensuring satisfactory outcomes during pregnancy, delivery, and the postpartum period.
The literature contains scant information on the prognosis of pregnancy in women with psoriasis, or on associated risk factors and comorbidities or therapeutic management. Most of the information available is based on case reports and series and data from pregnancy registries,3 whose purpose is to collect information on pregnancy and related complications and help identify possible teratogenic effects and evaluate the safety of exposure to particular drugs during pregnancy.
The aim of this review is to provide up-to-date information and to review the literature on the interrelationship between psoriasis and pregnancy and on current treatment recommendations for this special situation. Due to space constraints, biologic agents will be dealt with in a second part of this review.
Material and MethodsWe performed a systematic literature search to identify relevant studies. Our search yielded 362 publications using the terms “psoriasis and pregnancy” and 243 publications using the terms “psoriasis treatment and pregnancy”. We then searched for each treatment used in psoriasis in combination with the terms “pregnancy” and “lactation”. All of the references were scanned individually.
Immune and Hormonal Changes During Pregnancy and Their Effect on PsoriasisThe maternal immune system undergoes physiological changes during pregnancy to achieve immune tolerance towards paternal antigens expressed by fetal cells and thereby prevent rejection of the fetus. These changes, which occur both at the maternal-fetal interface and in the systemic circulation, are influenced by the effect of estrogens, progesterone, and cortisol, whose blood levels gradually increase over the course of pregnancy, giving rise to a change in helper T (TH) cell polarization that potentiates the humoral TH2 response and inhibits the cell-mediated TH1 response.4 Cytokines play a key role in the regulation of physiological and pathological processes such as pregnancy and psoriasis. During pregnancy, multiple cytokines are produced by different types of maternal and fetal cells in the uterus and the fetoplacental unit; the cells involved are not only immune system cells, but also other types of cells such as maternal decidual cells and fetal trophoblasts. The close relationship between the embryo and the endometrium and the placenta and the decidua is mediated by steroid hormones5 and diverse cytokines and chemokines, thereby ensuring sufficient cross-talk to ensure successful maintenance of pregnancy.6 During the formation of placenta, there is a clear tendency towards the formation of TH2 cytokines. Levels of these cytokines appear to progressively decrease during the second and third trimesters of pregnancy as the placenta completes its formation. In the third trimester, and particularly in the last weeks of pregnancy, the balance of cytokines is once again reversed towards a predominance of TH1 cells, which stimulate labor and protect both the mother and fetus against infection during and after birth.1,2
Numerous molecules are involved in implantation, and defective molecules rarely have a significant effect due to the complementary and compensatory action of other cytokines.7 Cytokine balance at the time of implantation is practically neutral. To date 2 molecules have been identified as playing a crucial role in implantation: interleukin (IL) 1, which is primarily produced by activated macrophages, and leukemia inhibitory factor, which is crucial for implantation and inhibits the differentiation of embryonic stem cells; it is produced by TH2 lymphocytes and the endometrial endothelium.6
An adequate cytokine environment is crucial during the early stages of embryo implantation and subsequent invasion of maternal uterine vessels by trophoblast cells, and it would appear that TH2 lymphocytes promote trophoblast anchorage, thereby favoring maintenance of pregnancy.8 TH2 cells produce cytokines such as IL-4, IL-5, and IL-13, which, through the activation of B cells and the production of antibodies, are responsible for the pathogenesis of several diseases such as lupus erythematosus.9 TH1 cells, on the other hand, produce proinflammatory IL-2 cytokines, interferon (IFN) γ, tumor necrosis factor (TNF), and IL-12, which are involved in cell-mediated tissue damage in certain autoimmune diseases, such as rheumatoid arthritis, Crohn disease, and psoriasis, among others.10 IFN-γ is the main mediator of the TH1 response, as it has a considerable inhibitory effect on TH2 response. These molecules are directly embryotoxic and complicate fetoplacental development through multiple mechanisms. Il-10 is an anti-inflammatory cytokine produced by TH1 and TH2 lymphocytes, and it downregulates proinflammatory cytokine production by TH1 lymphocytes and macrophages, favoring a TH2 response.11
In placenta, prostaglandin D2 and its metabolite, the cyclopentenone 15-deoxy-Delta(12,14)-prostaglandin J2 (15dPGJ2), acting through prostanoid D (DP1) receptors and the chemotactic TH2 receptor, CRTH2 or DP2, promote local accumulation of TH2 cells at the maternal-fetal interface12 and exert an anti-inflammatory effect through the inhibition of nuclear factor κB and the nuclear peroxisome proliferator-activated receptor (PPAR) by 15DPGJ2.13
The immune changes that take place during pregnancy, which favor TH2 activity, could explain why certain autoimmune diseases that involve TH1 cytokines, such as psoriasis and rheumatoid arthritis, tend to improve during pregnancy, while those involving TH2 cytokines, such as lupus erythematosus, tend to worsen.14
Likewise, multiple cytokine network dysregulation can lead to complications during pregnancy such as spontaneous abortion, premature birth, preeclampsia, and intrauterine growth restriction. Increased secretion of IFN-γ and IL-10, for example, has been associated with an increased risk of premature birth, while increased production of IL-6 and IL-1 at the implantation site has been linked to recurrent miscarriage.15
TNF has a dual, almost paradoxical, role in embryo development, as it is a potent activator of both apoptotic and antiapoptotic signaling cascades. It now seems clear that this cytokine not only promotes embryo destruction in the event of severe structural abnormalities, but also stimulates protective mechanisms that prevent against abnormalities that could compromise normal fetal development. In fact, several TNF single nucleotide polymorphisms (SNPs) have been associated with heart defects (tetralogy of Fallot or ventricular septum defect) and lower limb abnormalities.16 SNPs represent the minimum level of genetic differentiation between individuals, and in this post-genomic era, they are being increasingly used to identify genetic markers in complex diseases. As the regulatory regions of many genes are poorly characterized, attempts have been made to characterize coding area variations. TNF, however, provides a unique opportunity to study the distribution of SNPs in a genetic area where the regions involved in transcriptional regulation are well characterized.17
Hormonal changes during pregnancy play an important role in improving psoriasis, as they induce a state of immunological tolerance. Estrogens have both immunosuppressive and immunostimulatory functions. Maternal estrogens stimulate the production of IL-2, IL-10, and IFN-γ and inhibit the production of TNF in peripheral mononuclear cells. Three forms of estrogen are known: estradiol and estrone, which are derived from androgen and fetal precursors, and estriol, which is derived from fetal precursors. Estradiol and estrone predominate in early pregnancy, while estriol predominates in late pregnancy.18 Progesterone is primarily immunosuppressive, as it decreases proliferative T cell–responses and is a key factor in uterine immunosuppression. Because progesterone concentrations increase to a much greater extent than estrogens in pregnancy, it has been postulated that this change in the estrogen-progesterone ratio might be responsible for changes to the immune system during pregnancy. The shift towards the TH2 response in pregnancy occurs both in systemic circulation and at the fetal-maternal interface; progesterone inhibits the production of TH2 cytokines and induces TH2 activity, thereby contributing to adequate development of pregnancy.19 Prolactin and human placental lactogen also appear to have immunosuppressive functions, although their immunomodulatory role seems to be less well-established.5
In addition to contributing to TH2 polarization, hormonal changes during pregnancy appear to directly affect keratinocytes. It was recently demonstrated that these cells possess estrogen and progesterone receptors, which are specifically located in the cytoplasm of suprabasal keratinocytes.20 Progesterone receptor immunoreactivity is not related to hormones but rather to inflammation in the epidermis and dermis.20 Considering that progesterone acts as an immunosuppressant, it could be postulated that its receptor might be induced by unknown cytokines that would cause progesterone to increase or decrease the inflammatory process. This is an interesting field of research.
Course of Psoriasis During Pregnancy and Associated Risk FactorsAlthough pregnancy has an unpredictable effect on psoriasis, it is generally accepted that between 30% and 40% of women with psoriasis experience clinical improvements during pregnancy and a worsening of disease between week 4 and 6 postpartum; between 40% and 90% of women can experience a significant disease flare in the immediate postpartum period.21 One retrospective study of 91 pregnant women with psoriasis reported improvements in 56% of patients, worsening in 26%, and no changes in 17.6%.22
In another study, improvements were noted in 55% of pregnant women with psoriasis, worsening in 23%, and no noticeable changes in 21%.5 In the postpartum period, 9% of the patients experienced improvement, 65% experienced worsening, and 26% experienced no clinical changes in disease activity.
In most cases, the improvements observed in psoriasis during pregnancy occurred in the first 2 trimesters, both in the pregnancies studied and subsequent pregnancies.5
Boyd et al.14 postulated that improvements in psoriasis during pregnancy might be associated with hormone-mediated suppression of the immune system, with a key role played by progesterone, not only at the immunological level, but also through a direct effect on keratinocytic proliferation. This hypothesis contrasts with the results of the study by Murase et al.,5 in which improvements in psoriasis during pregnancy were linked to high levels of estrogen, with no correlation observed with progesterone levels.
The systemic inflammation that occurs in different immune-mediated diseases, such as rheumatoid arthritis, lupus erythematosus, inflammatory bowel disease, and moderate to severe psoriasis, may lead to poor prognosis and disease progression, with a particular risk of preterm birth and low birth weight.23 Increased levels of diverse proinflammatory cytokines (IL-6 and TNF) and inflammation biomarkers (C-reactive protein) in both maternal serum and cord blood during the pregnancy of women with psoriasis have been shown to result in preterm birth or small-for-gestational-age neonates.23 The coexistence of inflammation biomarkers (high-sensitivity C-reactive protein) and placental dysfunction (low levels of placental growth factor), which have been associated with trophoblast implantation defects, preeclampsia, and intrauterine growth restriction, have been linked to a 6- to 7-fold increase in the risk of premature birth.24 Cytokine imbalance can lead to endothelial dysfunction, resulting in systemic and placental vascular disease through the induction of platelet aggregation, intermittent vasospasm, and activation of the coagulation system.25 It has been postulated that placental vascular disease contributes to low birth weight, which is also a complication of preeclampsia, which, in turn, is associated with inflammatory activity and a subsequent increase in the same cytokines as in psoriasis.25
Several studies have attempted to link psoriasis and psoriasis severity to the development of gestational, fetal and/or perinatal complications. One case-control study of 145 pregnant women with psoriasis found a statistically significant association with several complications, such as hypertension, recurrent miscarriage, and cesarean delivery.26 Cohen-Barak et al.27 conducted a retrospective study in which they compared 68 pregnancies in 35 women with moderate to severe psoriasis with 237 pregnancies in 236 healthy controls to investigate the association between disease severity and gestational and/or fetal complications. They found that compared with the controls, women in the psoriasis group had significantly higher mean (SD) rates of spontaneous abortions (0.42 [0.58] vs 0.26 [0.63], P=.002), induced abortions (0.32 [0.60] vs 0.06 [0.25], P=.001), and large-for-gestational age neonates (3375 [543] vs 3247 [460] g, P=.03). They also found that pregnant women with psoriasis had a higher rate of pregnancy-induced hypertensive diseases (7.4% vs 2.1%, P<.05), premature membrane rupture (16% vs 5.5%, P<.008), and fetal macrosomia (13% vs 4.2%, P=.02). No significant associations were observed between gestational, fetal, or perinatal complications and either disease duration or use of systemic therapy during pregnancy.
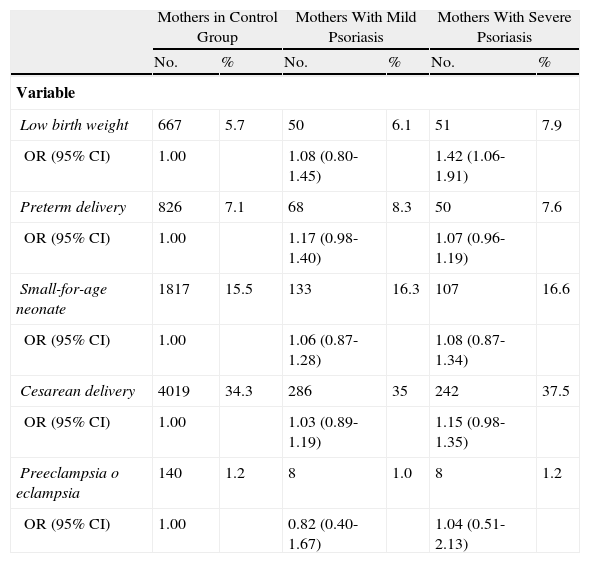
Yang et al.28 conducted an extensive study of the Taiwanese population to investigate the correlation between psoriasis severity and the risk of gestational and/or fetal complications. They compared 1483 pregnant women with psoriasis (classified as mild or severe) with 11 704 pregnant women without psoriasis. Of the women with psoriasis, 645 (44.1%) had been treated with phototherapy or systemic therapy in the 2 years prior to delivery and were included in the severe disease subgroup. The authors found that these women had a greater odds (odds ratio, 1.40; 95% CI, 1.04-1.89) of giving birth to low-birth weight infants than those without psoriasis. Although the data were adjusted for comorbidities such as diabetes mellitus and cardiovascular disease, the impact of systemic therapy during pregnancy could not be excluded. Nonetheless, the authors observed an increased risk of complications, such as preterm birth, low birth weight, cesarean delivery, and preeclampsia in pregnant women who received systemic treatment, and therefore concluded that the intrinsic risk could be only attributable to the systemic inflammation caused by psoriasis. Mild psoriasis was not found to be significantly associated with an increased risk of complications (Table 1).
Distribution and Odds Ratios (ORs) for Low Birth Weight, Preterm Delivery, Cesarean Delivery, Small-for-Age Neonates, Preeclampsia, and Eclampsia, Comparing 13167 Mothers With and Without Psoriasis (2001-2003).
| Mothers in Control Group | Mothers With Mild Psoriasis | Mothers With Severe Psoriasis | ||||
| No. | % | No. | % | No. | % | |
| Variable | ||||||
| Low birth weight | 667 | 5.7 | 50 | 6.1 | 51 | 7.9 |
| OR (95% CI) | 1.00 | 1.08 (0.80-1.45) | 1.42 (1.06-1.91) | |||
| Preterm delivery | 826 | 7.1 | 68 | 8.3 | 50 | 7.6 |
| OR (95% CI) | 1.00 | 1.17 (0.98-1.40) | 1.07 (0.96-1.19) | |||
| Small-for-age neonate | 1817 | 15.5 | 133 | 16.3 | 107 | 16.6 |
| OR (95% CI) | 1.00 | 1.06 (0.87-1.28) | 1.08 (0.87-1.34) | |||
| Cesarean delivery | 4019 | 34.3 | 286 | 35 | 242 | 37.5 |
| OR (95% CI) | 1.00 | 1.03 (0.89-1.19) | 1.15 (0.98-1.35) | |||
| Preeclampsia o eclampsia | 140 | 1.2 | 8 | 1.0 | 8 | 1.2 |
| OR (95% CI) | 1.00 | 0.82 (0.40-1.67) | 1.04 (0.51-2.13) | |||
Source: Adapted from Yang et al.28
Bandoli et al.2 investigated whether pregnant women with psoriasis had an excess of potentially modifiable risk factors for adverse pregnancy outcomes. They analyzed data from the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Project and compared the prevalence of certain risk factors between 170 pregnant women with psoriasis and 158 controls. Of the women with psoriasis, 128 had received biologic therapy at some moment during pregnancy. The authors found that patients with psoriasis, especially those with moderate to severe disease, were more likely to be overweight or obese prior to pregnancy (P<.0001), to smoke during the first trimester (P<.0001), and to be diagnosed with depression (P<.03). They also observed that the women with psoriasis were less inclined to be taking vitamin or folic acid supplements at the time of conception (P=.004).
The association between different psoriasis-related comorbidities and pregnancy- and delivery-related complications are summarized below25:
- -
Hypertension during pregnancy has been associated with low birth weight secondary to placental insufficiency, increased perinatal mortality, and preterm birth.
- -
Diabetes mellitus has been associated with fetal macrosomia, postnatal hypoglycemia, and congenital malformations, such as transposition of the great vessels.
- -
Obesity has been associated with macrosomia, certain congenital abnormalities, hypoglycemia, and low Apgar scores.
- -
Smoking has been associated with low birth weight, placental complications, and cleft palate.
- -
Alcohol consumption during pregnancy may result in fetal alcohol syndrome and low birth weight.
- -
Depression may be linked to a higher risk of preterm birth, low birth weight, and spontaneous abortions.
In a retrospective study, Lima et al.23 compared 162 pregnancies in 122 women with psoriasis with 501 pregnancies in 290 healthy women to investigate the association with the development of complications such as preterm birth (<37 weeks) and low birth weight (<2500g). They performed univariate and multivariate analyses, and adjusted for demographic factors (race, educational level, social background, number of previous pregnancies, etc.), associated comorbidities, and disease severity. The women with psoriasis had an increased risk of preterm birth and low birth weight in the univariate analysis (OR, 1.89; 95% CI, 1.06-3.39), and the association remained statistically significant in the multivariate analysis. No association was found between psoriasis and rate of spontaneous abortion, cesarean delivery, or preeclampsia.
As more information becomes available, we will gain a better understanding of the reciprocal relationship between pregnancy and psoriasis and the prevalence of potentially modifiable risk factors. This increased knowledge will help to optimize pregestational care in women with psoriasis and reduce the risk of gestational complications and fetal damage.
In the next section, we will review the scientific evidence available on the use of different treatments for psoriasis during pregnancy.
Treatment of Psoriasis During PregnancyThere is scant scientific evidence on the exact effect of certain drugs on embryonic and fetal development in pregnant women with psoriasis as clinical trials cannot be performed in this population for ethical reasons. Some of the current guidelines are based on retrospective studies and data from women who were taking a particular drug before knowing that they were pregnant.
When treating a pregnant woman with psoriasis, it is important to evaluate the severity and extent of disease, the potential risk to the fetus, the expected benefits of treatment, and the availability of safe and effective alternatives.
Topical and Nonbiologic Systemic TreatmentsTopical Corticosteroids. FDA Category CTopical corticosteroids are the mainstay treatment for many inflammatory skin disorders, including psoriasis, and they have been used in the setting for over 50 years. The literature contains many studies on the possible effects of oral corticosteroids on the fetus (see section on oral corticosteroids), and experimental studies have demonstrated fetal exposure to topical corticosteroids used by pregnant women (cited by Chi et al.29). Nonetheless, the doubts still surrounding the safety of topical corticosteroids in pregnancy cause reservations among both prescribers and patients and can jeopardize treatment. Percutaneous absorption of corticosteroids depends on numerous factors, including dose, excipient, treatment surface and application site, treatment duration, use of occlusive dressings, and number of applications.30 The extent of absorption varies between 0.5% and 7% in intact skin, but can increase in patients with inflammation and/or damaged skin.31 Bioavailability is also an important consideration. Hydrocortisone, for instance, which is considered the lowest-strength corticosteroid, can cause adrenal suppression when applied to severe skin lesions, and it should not be forgotten that pregnancy causes alterations to skin hydration and blood flow that can the alter the bioavailability of topical corticosteroids.32 In this last case, the effect of corticosteroids on the fetus will depend on transplacental passage, which is influenced by the activity of 11β-hydroxysteroid dehydrogenase (11β-HSDH), which is expressed in large quantities in the placenta and responsible for inactivation of cortisol.33 Metabolism of corticosteroid molecules in the placenta varies according to the molecule. Prednisolone crosses the placental barrier to the least extent (10%-12%),34 followed by hydrocortisone (15%), betamethasone (28%-33%), methylprednisolone (45%), and dexamethasone (67%).35–37 Fluticasone propionate and budesonide are not metabolized by 11β-HSDH, and would therefore be expected to reach the fetus in high concentrations.38
Chi et al.39 conducted a systematic review of the possible association between the use of topical corticosteroids in humans and certain congenital malformations, such as cleft lip, growth restriction, preterm birth, fetal death, low Apgar scores, and type of delivery. In most cases, they found no significant associations, even after controlling for the strength of the corticosteroids used. The abundant use of very high potency corticosteroids was linked to the appearance of cleft lip in one of the studies analyzed and growth restriction in another. Nevertheless, the authors of the systematic review concluded that the evidence to date was insufficient, as it was based mostly on case-control studies, and some cohort studies, most of which had insufficient sample sizes and numerous sources of potential bias.
In a population cohort study performed in the United Kingdom, 35503 pregnant women who had used corticosteroids at some time between 3 months before becoming pregnant to the end of pregnancy were compared with 48630 unexposed pregnant women.40 The corticosteroids were stratified according to potency. No significant association was observed between maternal exposure to topical corticosteroids and cleft lip. There was also no evidence of an increased risk of preterm birth or fetal death with the use of corticosteroids, regardless of potency. The authors, however, did find an association between fetal growth restriction and the use of high and very high potency corticosteroids, but not with low and moderate potency corticosteroids. A Danish retrospective cohort study of 22 480 pregnant women exposed to topical corticosteroids and 810156 unexposed pregnant women found no significant between-group differences on analyzing dose-response and potency-response relationships.41 In June 2011, Chi et al.42 published an evidence-based guideline on the use of topical corticosteroids in pregnancy that stated that low or moderate potency corticosteroids were preferable to high and very high potency corticosteroids. The guideline recommended that the latter group of corticosteroids should be used as second-line treatment for as short a time as possible and with appropriate obstetric monitoring (grade B evidence). The authors also stated that lipophilic corticosteroids should theoretically produce fewer effects on the fetus (level D evidence) because they caused fewer adverse effects and had the added advantage of once-daily application.
Topical Salicylic Acid. No FDA Pregnancy CategoryNo studies have been published on the use of topical salicylic acid in pregnant women.43 In one case report, however, its use was linked to premature ductus arteriosus constriction.44 In another recent study, topical 1% salicylate was linked to agnathia-otocephaly syndrome.45
Anthralin. FDA Category CNo studies have been conducted with anthralin in animals or humans.46
Tazarotene. FDA Category XTazarotene is an acetylenic retinoid that has proven to be effective in the treatment of mild to moderate plaque psoriasis when used at doses of between 0.05% and 0.1%. Its active form, tazarotenic acid, achieves measurable blood levels, but has not to date been found to have teratogenic potential in humans. Menter46 described the unpublished results of clinical trials performed by the manufacturer of topical tazarotene that described the birth of 8 healthy children in 6 women treated with this product, although no details were given on doses or duration of treatment. There was, however, mention of an increased rate of malformations and fetal death in rats and rabbits treated with oral tazarotene, the blood concentrations of which were much lower (1- to 13-fold) than those that would be expected in women treated with topical tazarotene. Consequently, unlike other topical retinoids such as adapalene or tretinoin, which are classified as category C in the FDA-assigned pregnancy categories, tazarotene is classified as category X. Considering that in practice the bioavailability of tazarotene following topical application is between 1% and 5% in patients with psoriasis,47 the potential teratogenic effect should be much lower than that of oral retinoids, which reach levels of up to 100 times higher in blood.
Calcipotriol. FDA Category CStudies in animals have shown an increased incidence of skeletal abnormalities and incomplete ossification of pelvic bones and phalanges of affected fetuses.48
Coal Tar. No FDA Pregnancy CategoryFranssen et al.49 undertook a retrospective study of the use of coal tar in 103 pregnant women; 59 did not use coal tar during pregnancy (19% had a spontaneous abortion and 5% had infants with congenital malformations); 21 possibly used coal tar during this period (fetal deaths, 5%; congenital malformations, 5%); and 23 used coal tar during pregnancy (spontaneous abortions, 26%; congenital malformations, 4%). The conclusion was that coal tar should be prohibited during the first trimester of pregnancy and limited during the second and third trimesters.
Tacrolimus. FDA Category CTacrolimus is a calcineurin inhibitor that is widely used as a topical treatment for atopic dermatitis. Thanks to its immunosuppressive properties and the fact that it does not cause skin atrophy, it has become a key corticosteroid-sparing agent. Its value has therefore been studied in other inflammatory skin conditions, such as psoriasis, in which it has shown good results, particularly in patients with facial and intertriginous forms of the disease.50 No studies to date have investigated the safety of tacrolimus in pregnant patients. It has, however, been demonstrated that tacrolimus is absorbed to a lesser extent than corticosteroids, and that repeated use on extensive areas for long periods of time is associated with low or undetectable blood levels of the substance, although absorption is greater in certain conditions with impaired skin barrier function.51 The use of oral tacrolimus in transplant recipients has given rise to numerous studies on the safety of this product during pregnancy. Jain et al.52 analyzed 37 liver transplant recipients treated with tacrolimus who became pregnant, and observed an increased risk of premature birth and low birth weight. Kainz et al.,53 in turn, on analyzing 100 pregnant transplant recipients, reported similar results to those seen in other studies of tacrolimus and other immunosuppressants, and concluded that the outcomes could be considered favorable. There have been reports of hyperpotassemia and elevated serum creatinine concentrations that resolved spontaneously in the children of mothers treated with oral tacrolimus.54 There have been no reports of congenital malformations associated with the use of oral tacrolimus. The above findings, combined with the fact that blood concentrations of tacrolimus following topical use in patients with atopic dermatitis are 7 to 17 times lower than concentrations following oral use, suggest that topical tacrolimus could be a safe treatment for pregnant women with psoriasis, particularly if they have facial and intertriginous lesions.54
UV-B Radiation. No FDA Pregnancy CategoryThe effectiveness of UV-B phototherapy in psoriasis is based on a wavelength of 290 to 320nm, with no need for additional topical or oral photosensitizers. The antiproliferative and immunosuppressive effect of UV-B radiation is essentially determined by inhibition of DNA synthesis through the formation of pyrimidine dimers and the release of prostaglandins and cytokines. Since it was introduced in the 1980s, narrowband UV-B therapy (311-313nm) has yielded slightly inferior results to those seen with psoralen plus UV-A (PUVA) therapy, but unlike PUVA, it is not associated with cancer risk or nausea and does not need to be combined with a systemic photosensitizer.55 According to El-Saie et al.,56 narrowband UV-B therapy appears to reduce serum folate levels in women. Pregnant women with psoriasis, thus, would have a double risk of low folate levels, and hence an increased risk of fetal neural tube defects. To minimize this risk, the authors recommend measuring serum folate levels before initiation of narrowband UV-B therapy, monitoring levels throughout treatment, and prescribing supplementation with folic acid at 5mg daily. Vun et al.57 published the case of a pregnant woman who developed generalized pustular psoriasis in the third trimester of pregnancy. Treatment was started with topical betamethasone with occlusive dressings, but improvement was minimal. The addition of UV-B therapy at 27 weeks resulted in clinical improvement and relief of symptoms. UV-B therapy, used alone or in combination with topical treatment, has therefore, been proposed as a good option for pregnant women who respond poorly to topical treatment only.
Systemic Corticosteroids. FDA Category CThe use of systemic corticosteroids, which are used to treat a wide range of conditions during pregnancy, has been associated with certain congenital abnormalities and premature birth. In a large retrospective study of 184 patients exposed to prednisone at doses of between 2.5 and 100mg/d during the first trimester of pregnancy, Park-Wyllie et al.58 showed a statistically significant association with premature birth, low birth weight, and congenital abnormalities, including cleft palate, anencephaly, atresia of the left ventricle, hypospadias, and pulmonary valve stenosis. Crowther et al.59 evaluated 1047 children born to 982 pregnant women exposed to betamethasone during pregnancy; 489 of the women were administered weekly doses of 11.4mg, while the other 493 received a single dose at week 24-28 of pregnancy. The authors observed higher rates of low birth weight in infants born to women treated during the third trimester and more attention problems at 2 years of age among children exposed to repeat doses of corticosteroids. It should be noted that systemic corticosteroids are considered a treatment of choice for impetigo herpetiformis, which is a form of pustular psoriasis that occurs during pregnancy, mainly during the third trimester.
Acitretin. FDA Category XFetal malformations have been reported in association with the use of acitretin during pregnancy. These malformations include meningomyelocele, facial deformations, syndactyly, absence of terminal phalanges, malformations of pelvis, ankle, and shoulder, cleft palate, cardiovascular malformations, low-set ears, decreased cranial volume, and alterations of the cervical vertebrae.60
Ciclosporin. FDA Category CCiclosporin, a potent T-cell inhibitor, remains one of the treatments of choice for moderate to severe psoriasis. In 1997, Lamarque et al.61 published a retrospective study of 629 pregnant women exposed to ciclosporin at doses of between 1.4 and 14mg/kg/d throughout pregnancy. They observed no significant differences with the general population in terms of the frequency of fetal death or congenital malformations. They did, however, find an increased rate of premature birth (44.5%) and low birth weight (44.3%) in the group of exposed women. Bar Oz et al.62 analyzed 15 studies on the use of ciclosporin in pregnant transplant recipients and reported an increased rate of premature birth. Armenti et al.,63 in turn, studied 392 pregnant transplant recipients treated with ciclosporin (unknown dose and duration of treatment), and did not observe an increase in birth defects. Finally, Edmonds et al.64 published a case report of a pregnant woman diagnosed with pustular psoriasis treated with ciclosporin 2.5-3mg/kg/d and prednisolone 40mg/d at week 21 who gave birth prematurely, with premature membrane rupture and low birth weight.
It should be stressed that the above studies involved transplant recipients with associated comorbidities (often severe) who were receiving multiple treatments, factors that in all likelihood contributed to fetal outcome. The results, therefore, cannot be truly extrapolated to patients with psoriasis. Ciclosporin can also cause hypertension and nephrotoxicity, which could result in fetal damage. Renal toxicity has been described in the fetuses of rats and mice, but no evidence of impaired renal function has been seen in humans, in particular mothers treated with ciclosporin during pregnancy.65 It would appear logical that ciclosporin could affect T-cell development. One study of peripheral blood in children exposed to ciclosporin in the uterus reported a worsening of T, B, and natural killer cell function up to the first year of life. However, none of the patients has clinical signs of immunosuppression.66 The FDA therefore included ciclosporin in its pregnancy category C in 2008, meaning that its use should be avoided in pregnant women, unless the potential benefits for the mother outweigh the potential risks for the fetus.
Methotrexate. FDA Category XMethotrexate and its analog aminopterin are 2 well-known folic acid antagonists that exert their effect though irreversible binding to dihydrofolate reductase. Effects include DNA synthesis inhibition and suppression of inflammation. Methotrexate was first used to treat psoriasis in 1972 and its use is now widespread in this setting thanks to the extensive clinical experience accumulated over the decades and its low cost. Accordingly, however, the literature contains numerous cases reports and series describing the effects of exposure to methotrexate during pregnancy, with it now being accepted that the drug can cause damage to the embryo.72 Transplacental passage of methotrexate became a focus of investigation following the detection of chromosomal aberrations in the children of mothers treated with this drug whilst pregnant. Al-Saleh et al.73 were the first authors to confirm the passage of methotrexate from mother to child through the placenta. Fetal aminopterin/methotrexate syndrome includes growth deficiency, microcephaly, hypoplasia of cranial bones, craniosynostosis, upswept frontal scalp hair, broad nasal bridge, prominent eyes, low-set ears, maxillary hypoplasia, epicanthal folds, short limbs, hypodactyly, and syndactyly.74,75 Feldkamp and Carey76 reported that the period of maximum risk was at between 6 and 8 weeks of pregnancy, and that a weekly dose of 10mg/wk was sufficient to give rise to fetal aminopterin/methotrexate syndrome. In an attempt to shed light on the subject, Hyoun et al.72 compared the proportion of birth defects in the children of mothers treated and not treated with methotrexate, and reported a significantly increased proportion of cases of pulmonary atresia, craniosynostosis, and limb defects in those exposed. Methotrexate has also been associated with altered spermatogenesis in the form of chromosomal aberrations, morphological anomalies, and altered sperm motility. Nonetheless, Beghin et al.,78 in a review of 42 cases of fetuses whose fathers had been exposed to methotrexate at the time of conception, stated that exposure did not appear to be linked to an increased risk of fetal alterations. In view of the above, methotrexate is contraindicated in the treatment of psoriasis in pregnant women. Although folic acid has been shown to reduce the incidence of cleft lip and neural tube, cardiovascular, and urinary tract malformations in infants born to women treated with other folic acid antagonists, its effect on methotrexate has not been studied.79
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that no private patient data appear in this article.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Ruiz V, Manubens E, Puig L. Psoriasis y embarazo: revisión (i). Actas Dermosifiliogr. 2014;105:734–743.