The therapeutic arsenal for hemangiomas in early childhood can now be considered to include oral β-blockers, mainly propranolol. These drugs are thought to act as vasoconstrictors, regulating angiogenic pathways and inducing apoptosis of vascular endothelial cells. Although infantile hemangioma is not among the approved indications for β-blockers, many specialized clinics will prescribe propranolol before resorting to corticosteroids. A dosage of 2mg/kg/d, is usually employed with a dosing interval of 8hours. Propranolol is safe, causing few side effects, although cases of hypoglycemia, hypotension, diarrhea, reflux, cold hands and feet, bronchospasm, and hyperkalemia have been described. Generally, these adverse effects have not had serious consequences. Prescription in PHACE syndrome is controversial. In all cases, a cardiologist should assess the patient before treatment begins, blood pressure should be monitored, and pediatric follow-up should be scheduled. This review covers our current understanding of the indications, clinical response, and adverse effects of propranolol, a drug has revolutionized our attitude toward infantile hemangioma and the way we approach therapy. Clinical trials under way are also reviewed.
Los ß-bloqueantes orales –principalmente el propranolol– se consideran una opción más en el tratamiento de los hemangiomas infantiles. Se ha sugerido que pueden actuar a través del efecto vasoconstrictor, mediante la regulación de las vías implicadas en la angiogénesis y ocasionando apoptosis de las células endoteliales. Aunque su uso no está aprobado para esta indicación, en muchos centros se indican antes que los corticoides. La dosis más empleada es 2mg/kg/día repartida cada 8h. Es un fármaco seguro con escasos efectos secundarios. Se ha descrito hipoglucemia, hipotensión, diarrea, reflujo, frialdad de manos y pies, broncoespasmo e hiperpotasemia, generalmente sin repercusiones graves. Su indicación en el síndrome de PHACES es controvertida. Antes de iniciar el tratamiento se recomienda en todos los casos la realización de una evaluación cardiológica, la determinación de la presión arterial y el seguimiento pediátrico. En esta revisión se evalúan los conocimientos actuales sobre las indicaciones, la respuesta clínica, los efectos secundarios y los ensayos clínicos en curso de esta modalidad terapéutica que ha revolucionado la visión y el abordaje de los hemangiomas infantiles.
Hemangiomas of infancy are benign vascular tumors that develop in 3 phases: in the first, the tumor proliferates, the second is a rest phase, and the third is marked by tumor involution. The unanimous opinion is that these hemangiomas should be treated in the proliferative phase under the following circumstances: vision is affected or might be, visceral organ involvement may become life-threatening, rapid growth leads to anatomical distortion that may resolve only partially and leave sequelae, an airway is affected, and the tumor is causing congestive heart failure.1,2 Although a wait-and-see approach to uncomplicated infantile hemangiomas persists, an interest in more active management has begun to emerge. A passive attitude might be justified by the benign nature of these tumors and their tendency to resolve spontaneously, but many patients still require intervention at the end of the period of involution if the tumor has not completely disappeared. The difficulty of predicting how long involution will take and how complete it will be justifies taking a more active approach to starting treatment at an early age.3
Treatment should be decided on the basis of individual circumstances, such as the size and location of the tumor, complications, the phase at the time of evaluation, the involvement of other organs, and psychological factors. Here we will discuss the novel and highly promising use of β-blockers to treat hemangiomas of infancy. Propranolol is proving very effective in this setting and its use is therefore growing. As a result, surgical intervention is usually needed only when involution has been incomplete and removal of residual tissue or other corrective measures are required.
Corticosteroids or β-Blockers?Oral corticosteroids have been the drugs of choice for treating complicated hemangiomas of infancy to date,4 but oral β-blockers, which lead to better overall outcomes with few adverse effects, will probably overtake them in the near future. Although some practice guidelines still emphasize corticosteroids for first-line therapy, few of the major referral centers currently prescribe corticosteroids before propranolol. Our protocol has specified β-blockers as first choice for the last 2 years.
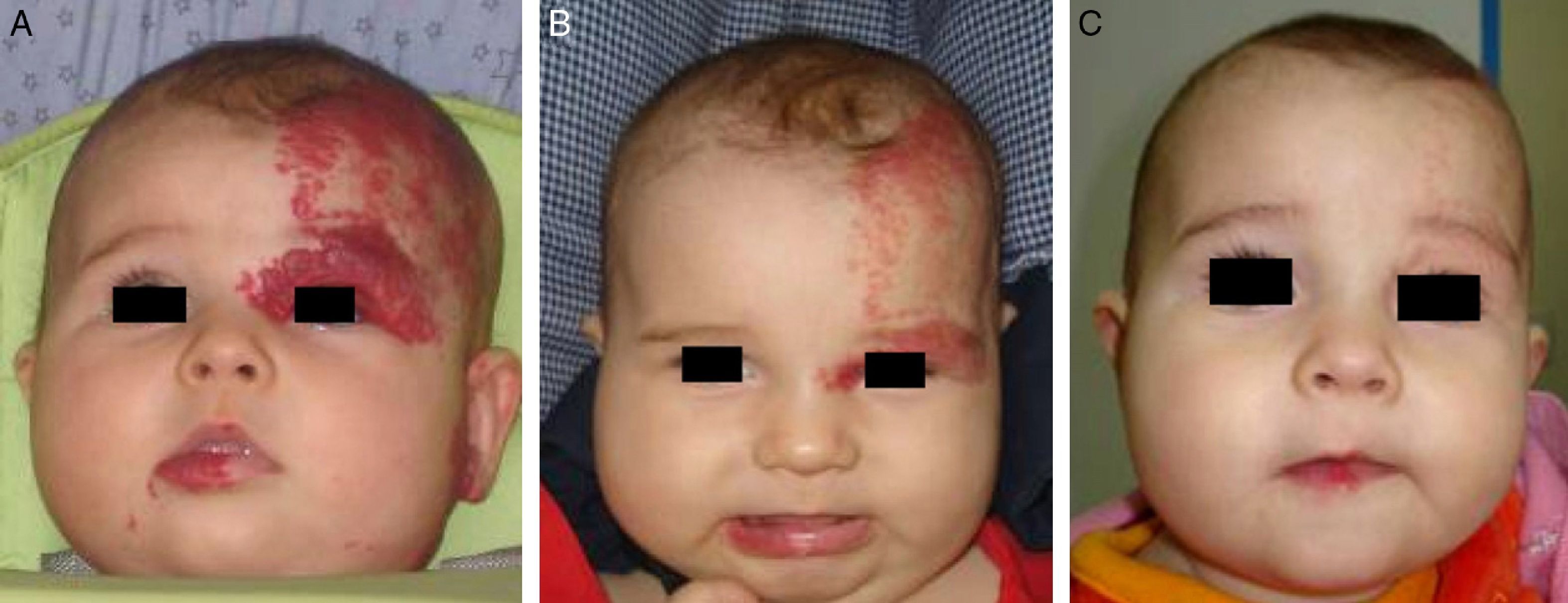
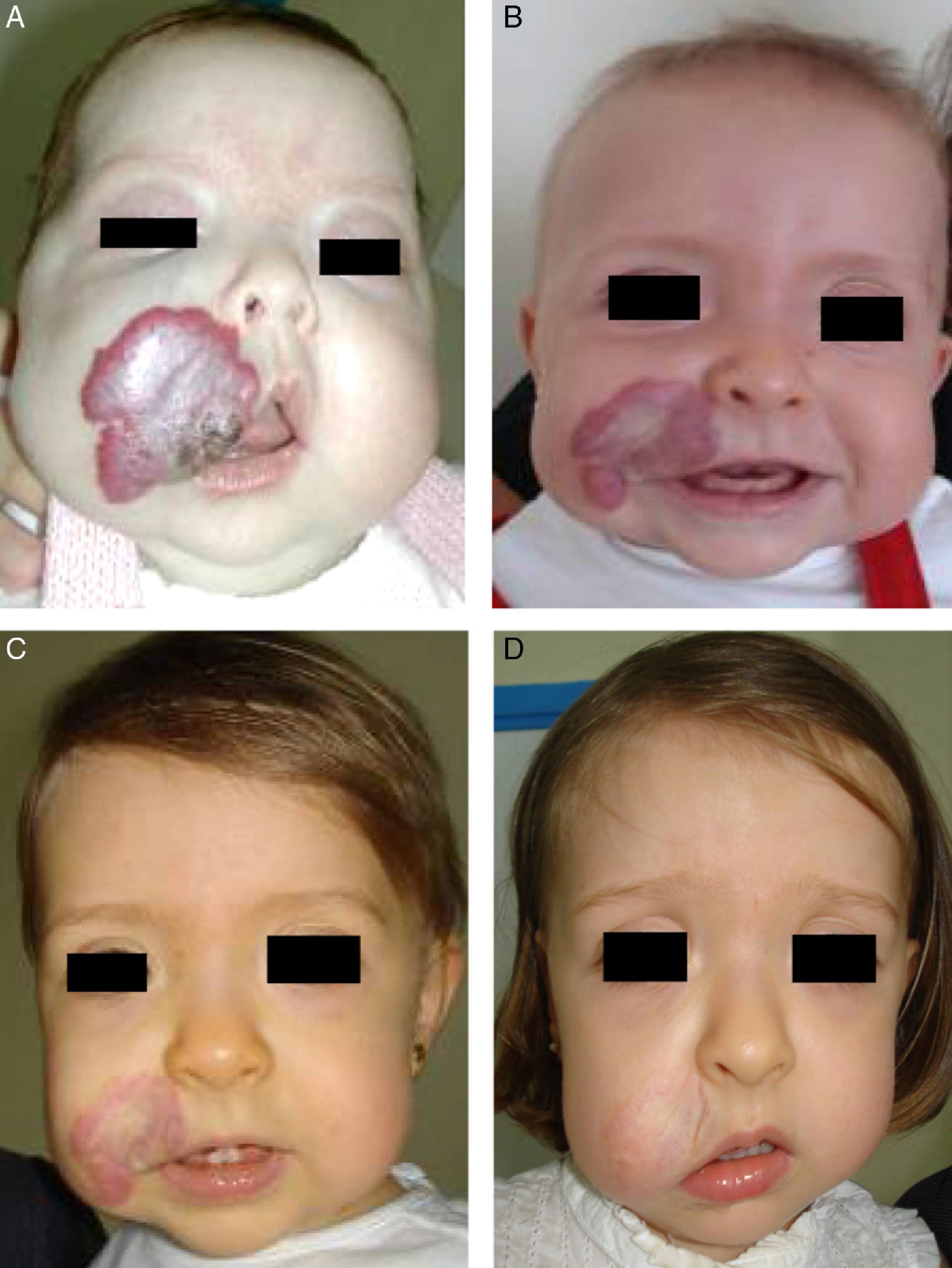
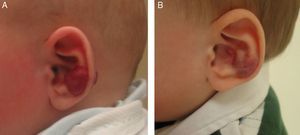
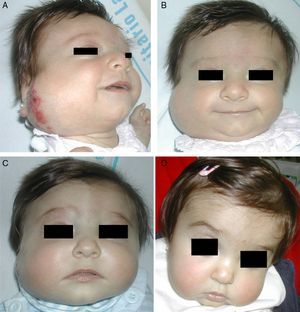
Accumulated clinical experience suggests that segmental hemangiomas (Fig. 1) respond better to propranolol than to oral corticosteroids; this is the reason we recommend propranolol as the first-line treatment. In focal hemangiomas (Fig. 2) outcome differences may be less pronounced; corticosteroid treatment can therefore still be considered, especially if the drug is to be injected directly into the tumor. Topical corticosteroids, such as 0.05% clobetasol propionate, have proven effective in small, superficial hemangiomas of infancy.5 It therefore seems more reasonable to prescribe topical clobetasol in such cases rather than propranolol, although topical β-blockers such as timolol can also be considered.
The treatment of hemangiomas of infancy has changed dramatically as a result of numerous reports of tumors that respond to propranolol after failing to respond to corticosteroids.6 With increased first-line use of β-blockers, it will soon be impossible to know whether the hemangiomas so-treated might also have responded to corticosteroids. Two valuable clinical trials are currently comparing these treatments, however7: one trial calls for prescribing corticosteroids versus propranolol and the other a combination of a corticosteroid plus either propranolol or placebo (Table 1). Oral corticosteroid-treated patients who later also take propranolol, while being weaned from the corticosteroids, respond satisfactorily, similarly to those who receive propranolol alone. Combined treatment, therefore, does not seem to make much sense.
Current Clinical Trials to Test β-Blocker Treatment for Hemangiomas of Infancy.
| Original Title | Objectives and Study Design |
| Double Blind, Randomised, Placebo-Controlled Study of Propranolol in Infantile Capillary Hemangiomas | - To determine the efficacy of 1 mo of treatment with propranolol (3mg/kg/d for 15 d, then 4 mg/kg/d for 15 days) vs placebo in infants <4 mo old for whom treatment is not imperative |
| Propranolol Versus Prednisolone for Treatment of Symptomatic Hemangiomas | - Oral propranolol 0.5 mg/kg, 4 times/d, for 4-6 mo |
| Propranolol vs Prednisolone for Infant Hemangiomas—A Clinical and Molecular Study | - Oral prednisolone, 1 mg/kg twice daily, 4-6 mo |
| Nadolol for Proliferating Infantile Hemangiomas: A Prospective Open Label Study With a Historical Control | - Oral nadolol, 0.5 mg/kg/d, taken in 2 fractions Weekly dose increases (0.5 mg/kg/d) if blood pressure and heart rate are acceptable, up to 2 mg/kg/d |
| Open-label, Uncontrolled Study of the Off Label Use of Propranolol for Infancy Hemangiomas to Identify Side Effects | - Initial treatment with 1 mg/kg/d of propranolol, increased after 24 h or longer if 3 sequential doses have been well tolerated without bradycardia or other adverse events- Propranolol treatment of complicated hemangiomas vs physical treatment (cryotherapy or laser therapy) in a comparison group of 16 patients- Propranolol 2 mg/kg/d in 3 fractions with or without concomitant therapy (surgery, laser therapy) |
| Topical Timolol 0.5% Solution for Proliferating Infantile Hemangiomas: A Prospective Double Blinded Placebo Controlled Study | - Topical 0.5% timolol in aqueous solution (2-3 drops) twice daily |
| A Comparative Study of the Use of Beta Blocker and Oral Corticosteroid in the Treatment of Proliferative and Involuting Cutaneous Infantile Hemangioma | - Oral propranolol, 2 mg/kg/d in 2 fractions for 60 d- Oral prednisone, 2 mg/kg/d, in 2 fractions for 60 d |
| Corticosteroids With Placebo Versus Corticosteroids With Propranolol Treatment of Infantile Hemangiomas | - Oral prednisolone, 1-2 mg/kg/d for 7 days followed by slow dosage reduction until withdrawal of treatment after 3 wk (2 mo of treatment in total), with placebo- Oral prednisolone, 1-2 mg/kg/d for 7 d followed by slow dosage reduction until withdrawal of treatment after 3 wk (2 mo of treatment in total), with oral propranolol, 2 mg/kg/d |
| A Randomised, Controlled, Multidose, Multicentre, Adaptive Phase II/III Study in Infants With Proliferating Infantile Hemangiomas (IHs) Requiring Systemic Therapy to Compare 4 Regimens of Propranolol (1 or 3 mg/kg/day for 3 or 6 Months) to Placebo (Double Blind) | - Oral propranolol, 1 or 3 mg/kg/d for 3 or 6 mo |
Source: http://clinicaltrials.gov/7
Another interesting new aspect of propranolol therapy is the response of hemangiomas during the involutional phase in children aged 2 or 3 years.8–10 Propranolol is effective in this phase whereas corticosteroids only act during the proliferative phase. Response is less marked with delayed treatment and is absent in some cases, but experience suggests that trying treatment for at least 3 months is reasonable in many cases in which tumor involution is incomplete or slow.
β-Blockersβ-Blockers antagonize the β-adrenergic pathway, blocking receptors in such organs as the heart, the pancreas, and the liver, as well as in peripheral blood vessels and bronchi. Some of these drugs (oxprenolol, pindolol, acebutolol, celiprolol) have intrinsic sympathomimetic activity, defined by an ability to both stimulate and block adrenergic receptors; thus, there is less risk of effects such as bradycardia and cold in the lower extremities. β-Blockers can also be grouped as cardioselective or not, or as lipophilic versus hydrophilic. Hydrosoluble agents (eg, atenolol, celiprolol, nadolol, and sotalol) penetrate brain tissue to a lesser degree and cause fewer sleep disorders. Propranolol is a noncardioselective β-blocker.
Relatively short-acting β-blockers are taken 2 or 3 times daily, while others—such as atenolol, bisoprolol, carvedilol, and nadolol—can be taken in a single daily dose. Oral propranolol is fully absorbed, reaching maximum plasma concentration 1or 2hours after intake on an empty stomach. Up to 90% of the drug is metabolized in the liver and the mean half-life is 3 to 6hours. The recommended dosing schedule is every 8hours. Propranolol is distributed rapidly throughout the tissues, reaching high concentrations in the lungs, liver, kidneys, brain, and heart. Protein binding is 80% to 95%.
The principal indications for propranolol are hypertension, angina, acute myocardial infarct, certain arrhythmias, heart failure and hyperthyroidism, but it is also used to alleviate anxiety, to treat glaucoma, and to prevent migraine. Given that infantile hemangioma is not yet listed as an approved use of propranolol in the summary of product characteristics, its prescription must be carefully explained to families and their written informed consent obtained.
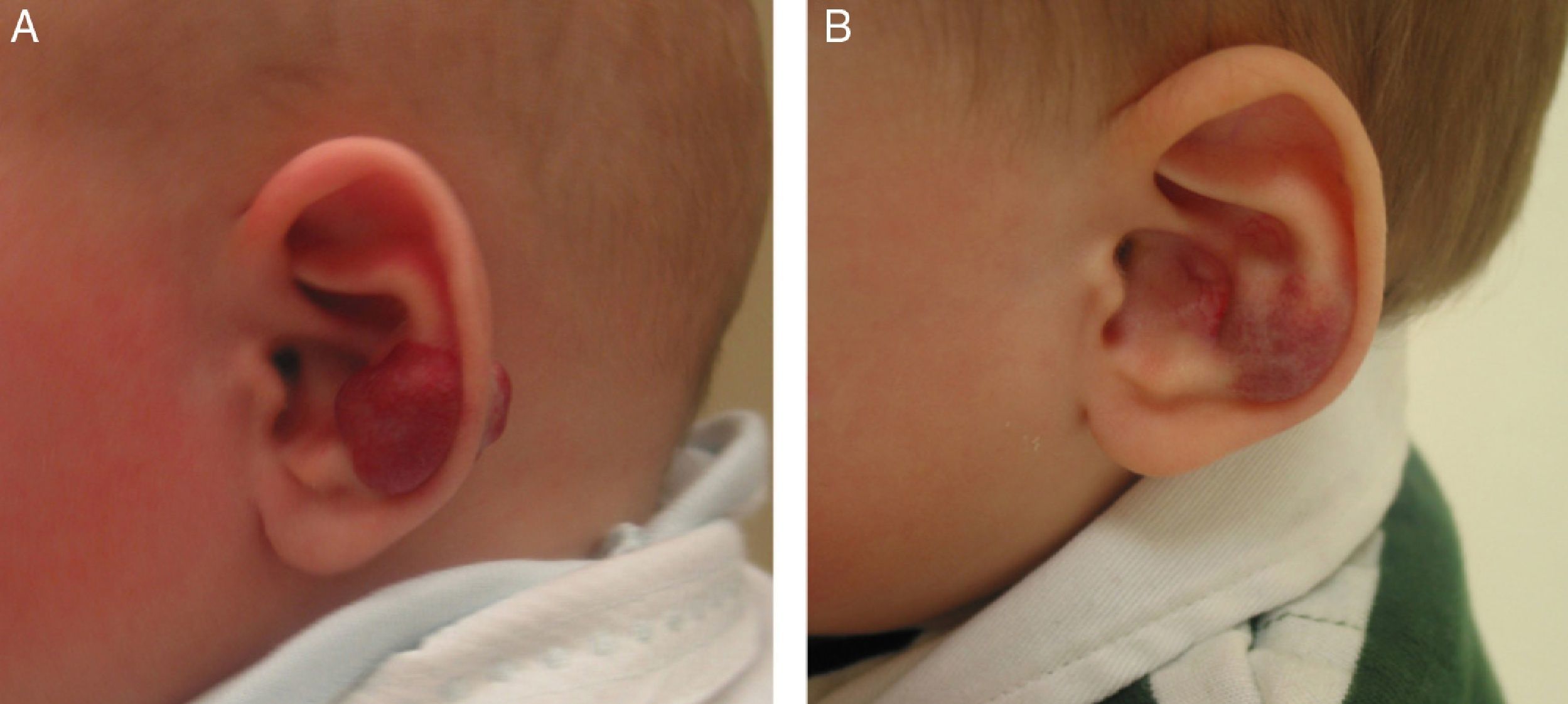
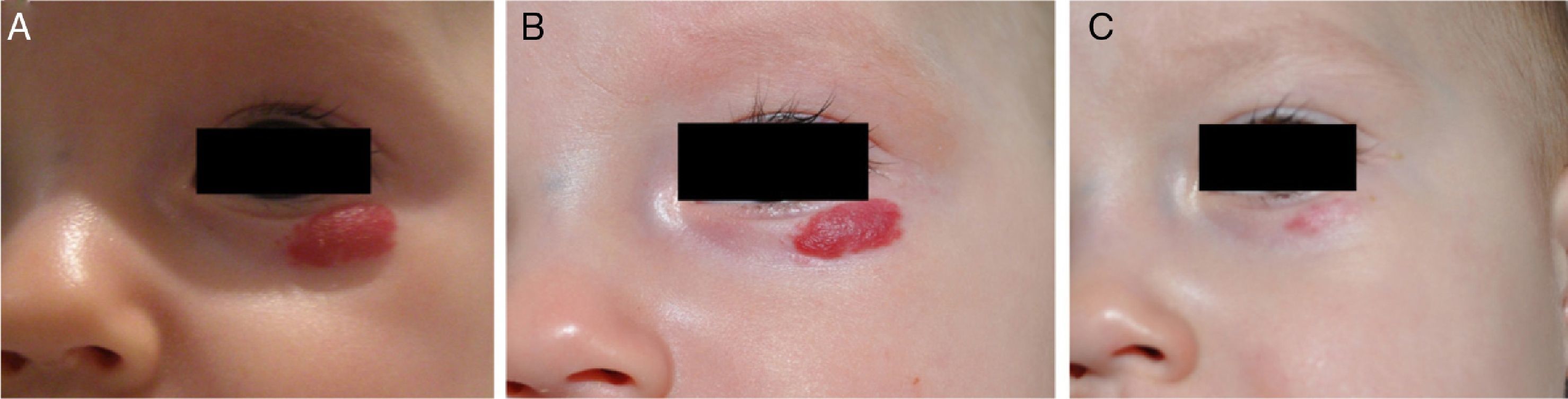
β-Blockers and Hemangiomas of Infancyβ-blockers (mainly propranolol) joined the therapeutic arsenal as soon as their effectiveness in hemangiomas of infancy was first reported in 2008.11 In most treatment centers they are considered the first line of therapy for this skin tumor even in the absence of official approval. The first report of use was in a series of 11 patients whose hemangiomas responded remarkably well to the drug,11 and that success was subsequently confirmed by publications of new case series and reports of single cases. Only hemangiomas of infancy seem to respond to propranolol (Fig. 3). Lack of response has been observed in congenital hemangiomas and tufted angiomas (J.C.L.-G., unpublished observations), as well as in other vascular lesions, such as pyogenic granuloma, which are negative on GLUT-1 staining.10
The ultimate mechanism of action of β-blockers in this setting has not been determined, but 3 possibilities have been suggested12: a) there may be a vasoconstrictive effect, reflected in the change in coloring; b) the expression of proangiogenic genes may be downregulated via reduced activity of the Raf-mitogen-activated protein kinase pathway (reducing the expression of factors such as vascular endothelial growth factor and basic fibroblast growth factor)13; and c) endothelial cell apoptosis may be induced.14
Current research seems to suggest that hemangiomas of infancy and retinopathy of prematurity share mechanisms involving a key role for vascular endothelial growth factor.15 The proposed use of β-blockers for retinopathy of prematurity16 is based on observations in an oxygen-induced animal model of the condition, in which topical timolol has been shown to prevent retinopathy in 40% of the cases and to mitigate its severity in remaining cases. Our need for a better understanding of the pathogenesis of this disease and its response to β-blockers has opened a new line of investigation that will also help us understand the mechanism that underlies their effect in hemangiomas of infancy.
Adverse Eventsβ-blockers slow heart rate and depress myocardial activity and are therefore contraindicated in patients diagnosed with second- or third-degree heart block. They should also be avoided in progressive or unstable heart failure, severe bradycardia, hypotension, sinus node diseases, and cardiogenic shock. These drugs can trigger an asthma crisis and prescription to asthmatics or in situations of bronchospasm should be avoided. Other effects that might appear are severe hypotension, bradycardia and congestive heart disease, digestive system disorders, shortness of breath, fatigue, sleep disorders (such as nightmares or insomnia), paresthesia, nausea and dizziness, depression and other psychological disorders, purpura, thrombocytopenia, hallucinations, exacerbation of psoriasis and alopecia, fatigue, and cold extremities. These drugs can also impair glucose tolerance and interfere with the metabolic and vegetative response to hypoglycemia. Other adverse effects that are the consequence of peripheral vasoconstriction are intermittent claudication and Raynaud syndrome. The frequency of many of these effects can be diminished by using selective β1-blockers.
Generally speaking, oral propranolol is safe in children and associated with few adverse events17; therapy needs to be withdrawn only rarely. Although unforeseen long-term side effects might still develop, we must remember that propranolol is a drug that has been used for some 50 years and has proven safe in children as well as in adults.18,19 We note, therefore, that the possible side effects of propranolol are currently being contrasted with those of oral corticosteroids. What tests do we order before prescribing oral corticosteroids for these patients? We do not check hypothalamic-adrenal functioning, nor do we monitor the immune system or check blood pressure systematically. Why should infants who are candidates for propranolol therapy be admitted to hospital and an echocardiogram ordered, blood pressure checked, and blood sugar determined? Propranolol toxicity is unquestionably mild: by mistake, a patient in our hospital ingested a dose that was 10-fold higher than prescribed for 3 days and developed only moderate hypotension. In contrast, oral corticosteroid therapy has been linked to a patient's death,20 and in our hospital we witnessed a death due to cryptococcal infection secondary to the immunodepressive effects of 9 months of corticosteroid therapy to treat a segmental hemangioma (J.C.L.-G., unpublished observations). Should such a death occur in relation to propranolol treatment, it would probably be the subject of a lead article in a high-impact journal. However, we do take heed of a recent commentary recalling the initial enthusiasm for interferon, a drug that is hardly prescribed now because of irreversible neurologic effects; propranolol use might still have a similar history.21
Few serious adverse events in infants treated with propanolol for hemangioma have been reported. Three patients developed severe hypoglycemia (associated with hypothermia in 2 infants).22 Cases of bradycardia and hypoglycemia21 or hypoglycemia alone23 have also been reported. In another case, an infant with multiple hepatic and cutaneous hemangiomas, without brain involvement, had a seizure caused by hypoglycemia.24 Hypoglycemia is known to be more common in the neonatal period and therefore its development must be watched for, but it does not seem to be dependent on propranolol dose as it has been reported in patients on low-dose regimens of 1 to 2mg/kg/d.22 Likewise, hypoglycemia does not seem to be a complication that develops on initiating treatment, as it has been detected in patients after as long as 9 months on propranolol. Although long periods of fasting have been blamed for hypoglycemia, a clear association has not been established: low blood sugar has been known to appear only 2to 3hours after a meal. Therefore, parents have been warned to avoid long periods of fasting, although frequent feeding does not seem to offer an infallible safeguard against this complication. It also does not seem to help to avoid dose increases at the start of therapy or to take propranolol in 2 or 3 fractions of the daily dose. Given the uncertainties surrounding propranolol use, we must wonder if it is really necessary to closely monitor these infants, even hospitalizing them for 48hours as some protocols have recommended. It seems more reasonable to educate parents to recognize the early signs of hypoglycemia (eg, sweating, trembling, tachycardia, hunger) and late manifestations (eg, excessive sleeping, lethargy, apneic episodes, seizures, poor appetite, loss of consciousness, and hypothermia).
Diarrhea and reflux have also been described among the side effects of β-blockers. Diarrhea has only been reported in 3 infants treated for hemangiomas at twice-daily doses of 1mg/kg25 and in 3 other infants in a series of 17 with periocular hemangiomas.9 Diarrhea may also be caused by the excipient used in certain preparations of the drug in maltitol-containing suspensions (the case of Syprol in the United Kingdom).25 Allergic reactions are rare and may also be related to a compound in the excipient. Reflux has been described in around 10% of cases in a series of 30 patients.26 Parents also report cold hands and feet. More worrying would be the possibility of hyperkalemia, which was recently described in 4-month-old girl under treatment for a hemangioma of the abdominal wall.27 High potassium concentrations were attributed in that case to massive lysis of hemangioma cells and impaired cell uptake of potassium due to the effect of the β-blocker.
Caries developed in incisors after administration of propranolol to a 10-month-old with a hemangioma on the lip.28 This complication might also be due to the excipient, which contained sucrose, or it may derive from reduced salivation, a consequence of adrenergic antagonism. Finally, excessive sleepiness was reported in around 27% of patients in a series of 30.26
Numerous publications report that propranolol is safe in these young patients, with no reports of death or serious complications,29 even in premature infants being treated for other conditions.30,31 Also supporting safety is the lack of any reports of serious complications when β-blockers have been used to treat hemangiomas of infancy.
What Dose Regimen Is Right and How Long Should Treatment Last?Dose regimens have varied from series to series. Most have called for 1to 3mg/kg/d of propranolol divided into 3 doses.6,32 We found a single report of using dose increments until 2mg/kg/d was reached in 2 patients, who took the drug twice a day and experienced no adverse effects such as hypoglycemia.33 Propranolol is usually taken orally; because a formulation appropriate for use in hemangioma of infancy is not yet available commercially, a solution in syrup is prepared. One report described injecting the drug intravenously for the first 5 days of treatment in 1 infant.34 Thus, no dosing regimen, including duration and monitoring periods, has yet been defined. We have observed no differences in clinical response with dosages of either 2or 3mg/kg/d, and have elected to begin with the 2-mg daily amount in a 3-dose regimen and maintain it if the clinical response is good. Experience thus far does not suggest that the rate of adverse effects rises when the initial dosage is 2mg/kg/d in comparison with a regimen of gradual increases building up to that level.
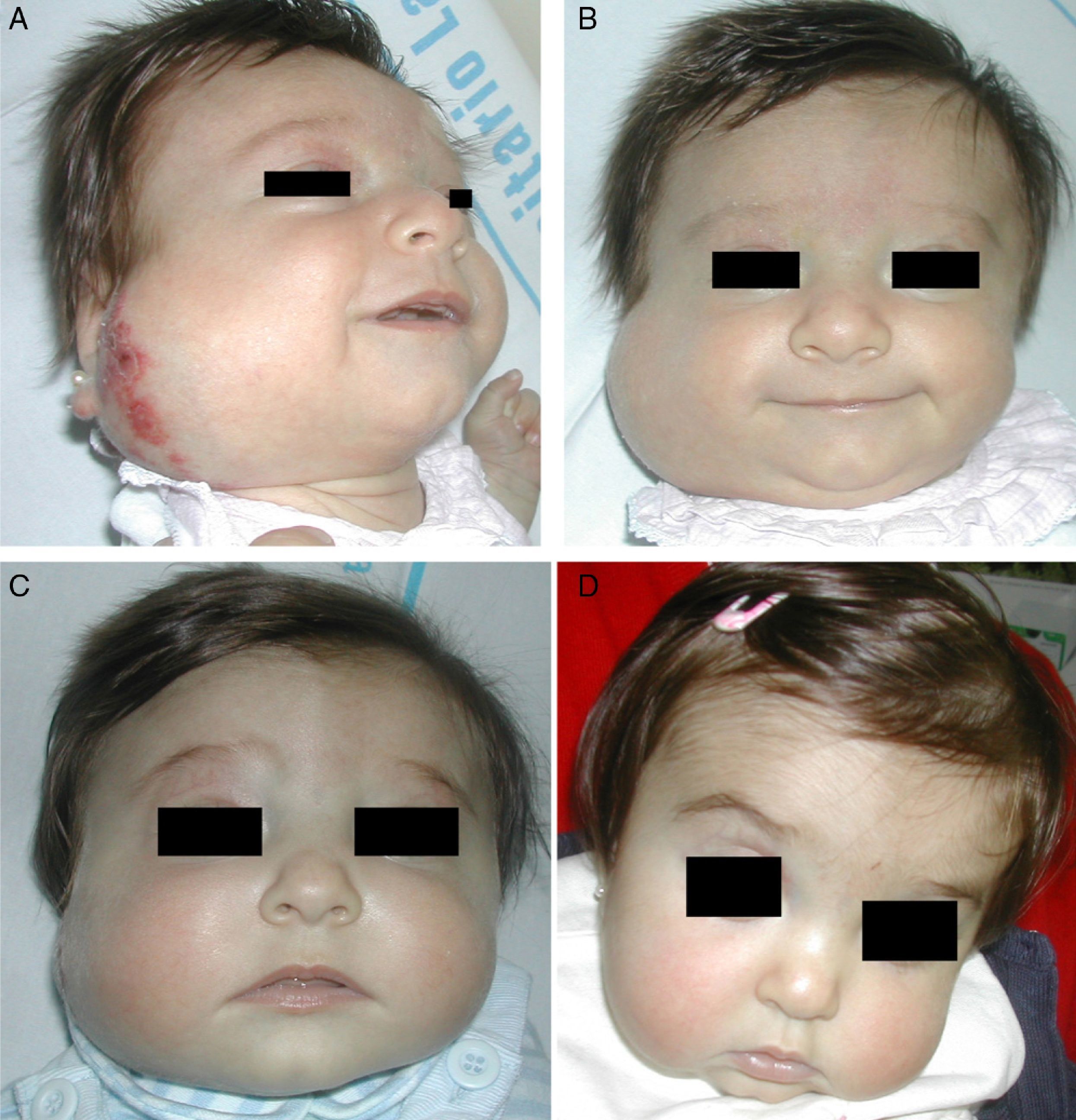
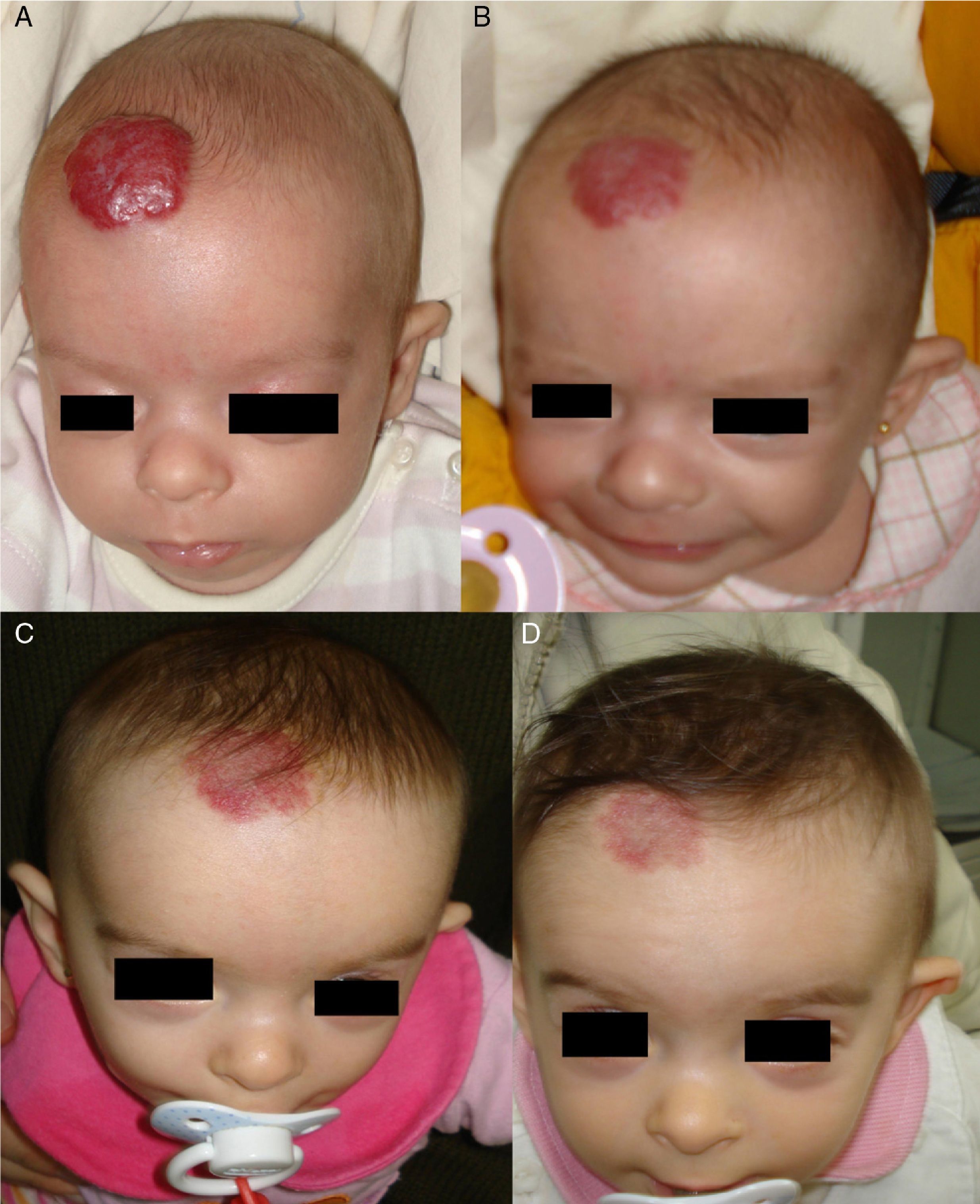
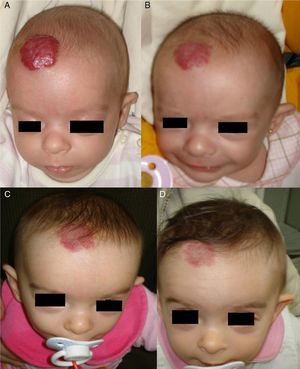
Regrowth sometimes occurs if treatment is withdrawn when the tumor is still in the proliferative phase.35 Propranolol can be restarted in these cases and the outcome is usually good,36 but the general recommendation is to maintain treatment until the proliferative phase has ended or until 12 months of age.32 Hemangiomas of infancy do not seem to disappear completely under propranolol treatment; however, the areas of telangiectasis that often remain can be eliminated later by pulsed dye laser treatment (Fig. 4). Experience with other β-blockers, such as acebutolol or nadolol, is more limited.37 In our dermatology department therapy is maintained until the age of 8 to 10 months or longer if the tumor continues to respond to treatment.
A, A 7-month-old boy with a scalp hemangioma. B and C, After propranolol treatment for 2 and 4 months (2mg/kg/d) improvement was substantial and included flattening of the lesion. D, The hemangioma did not fully disappear. Telangiectasis often persists and can later be removed by pulsed dye laser.
The sudden withdrawal of propanolol in angina pectoris can trigger or aggravate the angina and cardiac arrhythmia and even lead to acute myocardial infarction. In hemangioma of infancy, gradual weaning does not seem to be necessary, although some hospitals do include a period of dose reductions,6,34 theoretically minimizing the risk of a hyperadrenergic response. Some authors propose halving the dose for 2 weeks and then halving it again for 2 more weeks before stopping treatment entirely.6 Although propranolol is routinely withdrawn without a weaning period and without adverse effects, it seems prudent to advise gradual reduction so as to watch for regrowth and avoid any unforeseen problems. As tumor rebound occurs gradually, it is easy to decide to restart treatment in time.
MonitoringNo consensus has developed on how to monitor infants on propranolol. Nor do we know that monitoring is strictly necessary. Pretreatment evaluation by a pediatric cardiologist, unanimously believed to be essential, should include an electrocardiogram and assessment of blood pressure and heart rate. Some protocols also call for an echocardiogram, but we do not believe one is necessary if the results of the previous tests are normal. Some hospitals admit infants for the first 24 to 48hours of treatment for closer monitoring of blood sugar, blood pressure, and heart rate,17 but a benefit of hospitalization has not been demonstrated and this precaution seems to us and to others38 to be unnecessary. We recommend outpatient monitoring of blood sugar, blood pressure, and heart rate 48hours after start of treatment. We then check blood pressure and heart rate weekly in the first month and monthly thereafter. Periodic blood sugar tests are not necessary. As mentioned above, we believe it is more sensible to teach parents how to recognize the symptoms of hypoglycemia.
Propranolol or Another β-Blocker?Propranolol, a noncardioselective drug, has so far been the most commonly prescribed β-blocker in infantile hemangioma. The few groups that have used other β-blockers (nadolol and acebutolol) have reported similar results.37,39 As no trials have compared different β-blockers, there is no reason to prefer one over another.
Blanchet and colleagues37 reported good clinical response to acebutolol in 2 out of the 3 patients they treated for subglottic hemangiomas. Acebutolol is a selective β1-blocker that theoretically has fewer side effects than propranolol and is also easier to take, as dosing is every 12hours. The recommended regimen begins with 2mg/kg/d and continues with gradual increases until 8to 10mg/kg/d.37 Sold in France under the trade name of Sectral in a 40-mg/mL solution, this drug has been used to treat arrhythmia and hypertension in pediatric patients. Nadolol (sold as Solgol and used at doses similar to those of propranolol) has also been effective in our experience with over 20 patients (J.C.L.-G., unpublished observations). Given the history of our clinical experience with propranolol, it seems wise to continue using this β-blocker until information from additional trials becomes available.
Topical β-BlockersThe success of oral propranolol suggested that topical application might be effective in cases of small, superficial hemangiomas of infancy. In the first published series of 6 patients with such hemangiomas, good response to topical treatment was observed40 and has also been confirmed in isolated cases, such as that of a 4-month-old girl with a periorbital hemangioma causing blepharoptosis and covering the pupil.41 Substantial improvement (reduction of size, thickness, and coloring) was seen when 0.5% timolol maleate was applied twice a day for 5 weeks. In another series, 5 patients were treated with timolol gel.42 Response was remarkable in 1 infant and was partial in the remaining 4 infants, with no local or systemic side effects. Response was also good in another recent case of hemangioma with PHACE syndrome, in which a timolol lotion was applied.43
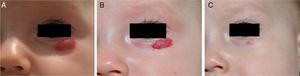
A randomized, double-blind placebo-controlled trial of 0.5% timolol in solution is now underway and will undoubtedly tell us more.7 In our experience with a small number of cases, results have been uneven to date: while some patients have improved significantly (Fig. 5) change has scarcely been evident in others. Formulations of 0.1% timolol gel and 0.5% timolol eye drops are available for ophthalmologic use in Spain. Topical β-blockers can be considered for treating uncomplicated small superficial hemangiomas of infancy; this option should be weighed alongside alternatives such as imiquimod and corticosteroids.5,44
Special CircumstancesUlcerated HemangiomasUlceration is the most common complication of infantile hemangioma, affecting between 5% and 16% of patients.1,45 As resolution may not occur spontaneously for several months, a physician should take an active approach to treating the ulcers. Oral corticosteroids and pulsed dye laser treatment have both been found to be effective.46,47 Topical imiquimod48 and becaplermin49 have also given satisfactory results when applied.
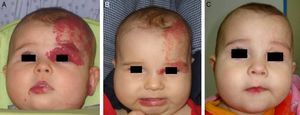
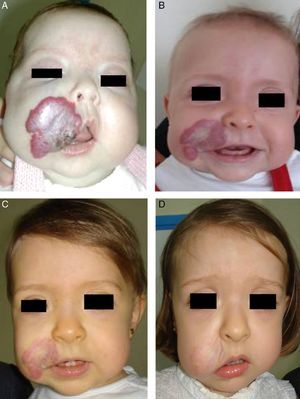
Likewise, oral propranolol has been used successfully in this setting,6,36,50,51 although not all ulcerated hemangiomas respond and some may even worsen6,52 under this treatment (Fig. 6). In a series of 30 infants with ulcerated hemangiomas, 10 with small lesions responded well, but the results for deeper ulcers were less satisfactory.6 In another recent study of 33 infants with this complication, a mean of 4.3 weeks was required to cure the ulcers of 30 patients.53
A, The hemangioma of this 2-month-old baby girl affected the parotid, cheek, and lip, with associated ulceration. B and C, Clinical response was highly satisfactory after 1 and 3 months of treatment with oral propranolol (2mg/kg/d). D, At the end of 9 months of treatment, there is redundant, atrophied tissue and slight deformity of the facial structure that can be treated with laser surgery.
Among the most important hemangiomas to treat early are those located near the eye. Even 2 weeks of impaired vision can lead to irreversible damage. The treatment of choice until recently was oral or intratumoral corticosteroids. However, the possibility of embolization54 after injection, causing occlusion of the retinal artery followed by blindness, has led to reaction against intratumoral injection.
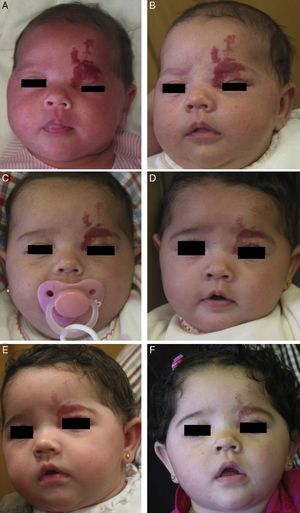
Propranolol is thus a good alternative for treating periorbital hemangiomas of infancy (Fig. 7). Most articles report rapid response, with remarkable reduction in size within 48to 72hours of starting treatment.55,56 In a series of 17 patients with periorbital hemangiomas, the tumor shrank after only a month of treatment in 82%.9 The authors did not say so, but it can be inferred that response differed according to whether the hemangioma was focal, segmental, or undetermined. In another series, response was observed in all 10 infants treated with 2mg/kg/d of propranolol. Furthermore, the amblyopia of 5 of the infants improved substantially in 3 of them.56 Outcome was good in all 4 patients in another small series in which no side effects occurred during treatment that started with low doses of 0.1to 0.25mg/kg/d and increased to 2mg/kg/d.8 Propranolol was even effective in a patient who started treatment at the age of 2 years, 5 months.
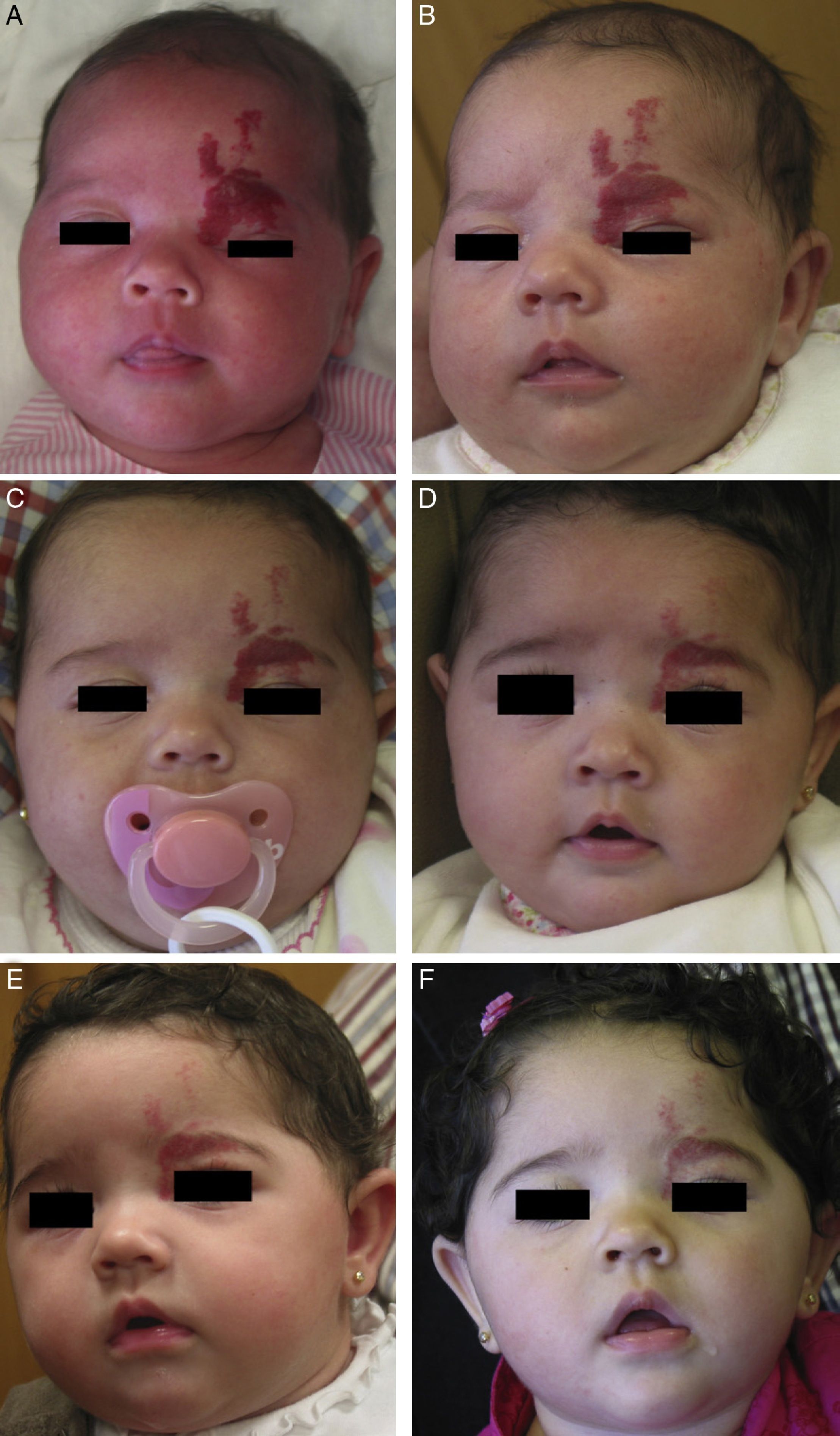
A, Infant girl at 6 weeks with a rapidly growing segmental hemangioma on the forehead and eyelid. Ptosis was marked and progressive. On magnetic resonance the position of the entire eye could be seen to be shifted due to retrobulbar growth of the hemangioma. Oral propranolol treatment was started at a dosage of 2mg/kg/d. B, Improvement could already be seen at 72hours. C-F, Under this treatment the clinical course was highly satisfactory at 1.5, 3, 4, and 6 months, respectively.
Facial and cervical hemangiomas, which may compromise the upper airway, are usually superficial, unilateral, and subglottic.57 To date, corticosteroids have been the first-line treatment, although a tracheotomy becomes necessary in a large percentage (40%) of cases. Carbon dioxide laser therapy can also be used.58
Various authors have reported propranolol to be effective in the treatment of upper airway hemangiomas, most of which have been subglottic, and for most it has been the first treatment chosen.59–61 Clinical improvement was observed in all 14 patients in a series described by Leboulanger and colleagues39: airway obstruction was evident in only 22% after 2 weeks of treatment and in 12% after 4 weeks.
Resistance to propranolol has been observed in isolated cases. Leboulanger and colleagues39 reported relapse when propranolol was stopped after 1 month in a patient, and when treatment was restarted resistance was noted. In another patient whose airway was initially 95% occluded, 50% reduction was observed after 2 weeks of propranolol treatment; however, obstruction increased again to 80% after several months in spite of treatment with 2mg/kg/d.62 These patients did not improve when the dosage was increased.
In most reports, infants with airway hemangiomas have also been receiving concomitant corticosteroids,10 but remarkable improvement was recently reported in 2 infants taking only propranolol from the start of treatment.33 Combining the 2 therapies appears to be unnecessary.
Finally, we wish to highlight the case of a 12-month-old who required β-mimetic drugs during cardiac surgery. He had experienced good response to 6 months of treatment with propranolol and was already on a low and decreasing dose regimen (0.5mg/kg/d) at the time of surgery. Tubes could not be removed after surgery and a tracheotomy was required. On examination, 95% of the airway was found to be occluded. Increasing the propranolol dose to 2mg/kg/d reduced the stenosis again and after 1 month of treatment only 5% of the airway remained obstructed.37
Good response to acebutolol, without adverse effects, has also been described.37,39 Two out of 3 patients in 1 report responded satisfactorily to a regimen that started with 2mg/kg/d, taken in 2 doses, and was later increased to 10mg/kg/d.37
Hemangiomatosis and Visceral HemangiomasWhen multiple hemangiomas are distributed widely over the surface of the body, the condition is termed hemangiomatosis. When visceral organs are involved, the most likely location for a hemangioma is the liver. When a hepatic hemangioma is found in association with hemangiomatosis, an infant is at high risk of heart failure63 and should be monitored regularly to detect complications requiring prompt treatment. Fortunately, the prognosis in these cases will surely change for the better, given the satisfactory response to propranolol that has been observed in some patients, in whom tumors have disappeared completely and the associated heart failure and hypothyroidism have also resolved.64 There have also been reports of cases of diffuse hepatic hemangioma that have responded rapidly and definitively to propranolol, after prior treatment with corticosteroids, vincristine, interferon, and cyclophosphamide.65,66 Finally, life-saving treatment in isolated cases of mediastinal, subglottic infantile hemangioma have also been reported.67
Propranolol in Patients With PHACE SyndromeFor a diagnosis of PHACE syndrome it is not necessary to observe all the characteristic findings: tortuosity of large posterior (P) cerebral vessels, large facial hemangiomas (H) accompanied by arterial (A), cardiac (C) and eye (E) anomalies.68,69 In an estimated 70% of PHACE syndrome cases, the infant has only extracutaneous manifestations. Dandy-Walker malformation has been described among the cerebral abnormalities, along with structural arterial abnormalities such as aneurysmatic dilatations and abnormal branching of the internal carotid artery.68 Cardiac defects and aortic malformations, such as coarctation, may be present in a third of these patients.70
The use of propranolol in infants with PHACE syndrome is controversial as the drug may lead to potentially dangerous complications such as cerebral infarct. Propranolol is not contraindicated in patients with vascular stenosis, but ischemic strokes resulting from hypotension or bradycardia secondary to the β-adrenergic blockade are possible if this treatment is used when PHACE syndrome involves arterial malformations with reduced flow.6,71 This complication has not been described to date, however, although it must be admitted that the number of these patients treated so far is very small.39,72 One very interesting study that is relevant to this controversy looked at cerebral perfusion by single-photon emission computed tomography, a technique that has proven useful in neurovascular conditions such as Sturge-Weber syndrome.73 When 7 infants with PHACE syndrome took 2mg/kg/d of propranolol, the authors observed normal uptake in frontal and temporal regions after 3 or 6 months in spite of malformations that had been identified by magnetic resonance angiography. Symptoms and hemangioma size also improved significantly. Another series included an infant with PHACE syndrome with aortic coarctation and absence of the right vertebral artery; this patient also responded well to propranolol.6 In conclusion, when PHACE syndrome is suspected, a cardiologist should perform a complete diagnostic work-up and neuroimaging studies should be ordered to confirm the diagnosis before starting propranolol treatment. Thus far, there is no reason to assume that the incidence of stroke in infants with PHACE syndrome is related to the treatment chosen; rather it would be attributable to the degree of cerebrovascular involvement. In fact, corticosteroids and interferon are used indiscriminately in PHACE-syndrome patients in spite of the antiangiogenic effects on the hemangioma and the brain, yet these treatments are not reported to increase the incidence of stroke.
Conclusionsβ-Blockers will probably become the treatment of choice for hemangiomas of infancy in the near future, but many points remain to be discussed. The optimal dose regimen, the type of β-blocker that is most effective, and the type of monitoring required during treatment, among other concerns, must still be studied. Until we have more information based on clinical experience and trials, we should be particularly cautious when prescribing. Given the excellent responses to these drugs to date, however, the indications will certainly be increasing. Parents must be warned that β-blockers are not yet approved for hemangiomas of infancy—and it is helpful to mention that corticosteroids are not so-approved either—and their written informed consent must be obtained. Although adverse effects have been described in isolated cases, we can say that β-blockers are safe in infants with these tumors. Publications describing ever larger series are confirming the absence of adverse events,74 even in low-birth-weight newborns.75 Until the results of clinical trials become available, the protocols for monitoring infants during treatment will continue to vary from hospital to hospital. In our opinion, outpatient follow-up by a pediatrician seems to be the wisest approach. We also recommend referring parents to specialists in vascular abnormalities for diagnostic confirmation and prescription of this treatment. Diligent follow-up and comprehensive treatment should be provided, given that some of these children may require additional laser or surgical treatment.
Conflicts of InterestDr Juan Carlos López Gutiérrez is principal investigator of the multicenter adaptive phase 2/3 randomized controlled trial of multiple dosages of propranolol in children with hemangiomas, funded by Pierre Fabre (registry number V00400SB201).
Ethical ConsiderationsData protection. The authors declare that they followed their hospitals’ regulations regarding the publication of patient information and that written informed consent for voluntary participation was obtained for all patients. None of the children photographed for this article were participants in the trial.
Right to privacy and informed consent. The authors obtained informed consent for the mention of patients in this article. All consent forms are in the possession of the corresponding author.
Please cite this article as: Sánchez-Carpintero I, et al. Propranolol en hemangiomas infantiles: eficacia clínica, riesgos y recomendaciones. Actas Dermosifiliogr. 2011;102:766-779.