Recent reports have described the successful use of propranolol to treat severe hemangiomas of infancy. The few case series that have been reported, however, have included only a small number of patients. The aim of this study was to describe the results of oral propranolol treatment for severe hemangiomas of infancy in terms of treatment outcome and the occurrence of adverse events.
Patients and methodsA descriptive, observational study was undertaken in a series of children with severe hemangiomas of infancy seen in the pediatric dermatology unit at Hospital Universitario Virgen del Rocío in Seville, Spain between July 2008 and December 2009. Patients were included if they had hemangiomas in the proliferative phase or involuting lesions with substantial residual deformity. All children were treated with oral propranolol (2mg/kg/d) and followed until September 2010. Epidemiologic characteristics were analyzed along with treatment response at 3, 6, 9, 12, and 18 months; adverse events were also recorded at those times.
ResultsThirty-six hemangiomas were treated in 28 patients. Propranolol treatment was effective in all cases, with a good or complete response in 88.2% at 6 months. Effects were apparent within a few hours of treatment, which was effective in both growing and involuting hemangiomas. In ulcerated hemangiomas, the mean healing time was 61days. Adverse events were mild and self-limiting. Only 2 patients discontinued treatment due to hypotension.
ConclusionsIn the majority of cases, oral propranolol produced rapid and sustained improvements in hemangiomas of infancy and shortened the natural course of the disease with few side effects. However, no significant reductions in symptoms or healing time were observed in ulcerated hemangiomas.
Recientemente se ha comunicado el éxito del tratamiento con propranolol para hemangiomas infantiles (HI) graves. Existen escasas series publicadas con reducido número de pacientes. El objetivo del presente estudio fue conocer la efectividad y seguridad de propranolol oral para HI graves.
Material y métodoEstudio observacional y descriptivo de una serie de niños con HI graves en fase proliferativa, o en fase involutiva si presentaban importante deformidad residual, que acudieron a la Unidad de Dermatología Pediátrica de nuestro hospital desde junio de 2008 hasta diciembre de 2009 y fueron tratados con propranolol oral (dosis de 2mg/kg/día). Fueron seguidos hasta septiembre de 2010. Se analizaron las características epidemiológicas, la respuesta al mes, 3, 6, 9, 12 y 18 meses y se registraron los efectos adversos.
ResultadosSe trataron 36 HI en 28 pacientes. El tratamiento con propranolol fue efectivo en todos los casos, con respuesta completa o buena en el 88,2% de los casos a los 6 meses de tratamiento. El efecto fue evidente en las primeras horas tras instaurar el tratamiento, siendo útil tanto en fase proliferativa como involutiva. En HI ulcerados el tiempo medio de cicatrización fue de 61 días. Los efectos adversos fueron leves y autolimitados. Sólo dos pacientes discontinuaron el tratamiento por hipotensión.
ConclusionesEl propanol oral induce una mejoría rápida y mantenida en la gran mayoría de los HI, acortando considerablemente su evolución natural y con escasos efectos secundarios. En HI ulcerados no observamos una reducción significativa de la sintomatología o tiempo de cicatrización.
Hemangioma of infancy is a benign self-limiting vascular tumor. Although it is estimated to affect 3% to 10% of children during the first year of life,1–5 a significant percentage of these lesions are associated with marked morbidity.2–4,6 No well-studied or approved systemic treatment is available. Oral prednisolone (2-5 mg/kg/d) is considered a first-line treatment,7–9 yet only 1 small randomized controlled clinical trial has compared this regimen with intravenous methylprednisolone.8 Systemic corticosteroids can prove effective in cases of high-risk lesions; however, treatment response is variable and the side effects are insidious, difficult to monitor, and potentially severe.10 Other drugs used to treat high-risk lesions could prove more harmful and have potentially more severe side effects, with even more uncertain results.1,5,10 These include vincristine, interferon alfa, and cyclophosphamide.10–13 Propranolol is a β-blocker that has been used for several years to treat heart conditions. The incidental finding of a rapid regression of hemangioma in a patient with obstructive hypertrophic cardiomyopathy who had received oral propranolol (3 mg/kg/d) pointed to a possible therapeutic effect of this agent on hemangioma of infancy.14 A recently published series of pediatric patients with hemangioma of infancy revealed a marked improvement after treatment with oral propranolol.14 This and other studies have led pediatric dermatologists to consider oral propanolol as an option for the treatment of high-risk hemangioma of infancy. At present, however, there are no studies comparing propranolol with alternative drugs, and the largest published series comprises only 32 patients.14–19 Furthermore, no case series on the use of propanolol to treat this disease have been published in Spain. Although propranolol is a well-known and widely used drug, many doubts still surround its use for the treatment of hemangioma of infancy, for example, with regard to optimal dose, duration of treatment, and relapse after discontinuation. Consequently, until a controlled clinical trial can be performed, it is of paramount importance that local case series be reported in order to broaden our experience with the use of propranol to treat this tumor.
The objectives of the present study were to determine the effectiveness and safety profile of oral propranolol solution for the treatment of hemangioma of infancy.
Materials and MethodsWe performed a descriptive, observational study in which we reviewed all patients with hemangioma of infancy referred to the Department of Pediatric Dermatology at Hospital Universitario Virgen del Rocío in Seville, Spain over an 18-month period (July 2008 to December 2009). Follow-up continued until September 30, 2010. The criteria for initiating propranolol for the treatment of hemangioma of infancy in the proliferative phase were risk of functional impairment, local complications, or deformity and age more than 1 month. In the case of involuting hemangioma, the more residually deformed lesions were treated in order to accelerate the natural course before surgery.
Patients with suspected PHACE syndrome were hospitalized for additional investigations (cranial magnetic resonance imaging, cerebral magnetic resonance angiography, abdominal ultrasound, echocardiography, baseline electrocardiogram [ECG], blood biochemistry, complete blood count, and evaluation by a cardiologist and an ophthalmologist). Once these had been performed, treatment with propranolol was started during hospitalization. Blood pressure (BP) and heart rate were monitored every 8 hours, blood glucose every 12 hours, and ECG daily for 2 days. The remaining patients underwent a study before initiation of treatment (ECG and evaluation by a pediatric cardiologist, BP and heart rate, blood biochemistry, and complete blood count). Outpatient treatment was then started, with monitoring of BP and heart rate every 3 days during dose escalation (12 days) and subsequently every week. Monitoring was performed at the patient's health center under the supervision of the area pediatrician.
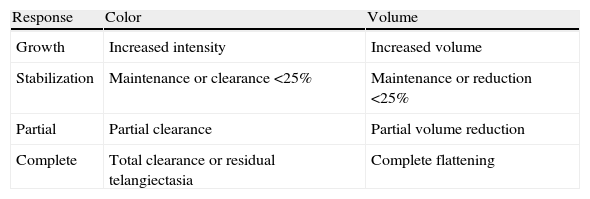
Once parental informed consent was obtained, treatment with oral propranolol suspension was started at 5 mg/mL. The initial dose was 0.5 mg/kg/d (0.25 mg/kg/d taken in 2 doses), with an increase of 0.5 mg/kg/d every 3 days, until a final dose of 2 mg/kg/d was reached. Patients included in the initial stages of the study were reassessed at 1, 3, 6, 9, 12, and 18 months. Patients included in the final stages of the study were reassessed at 1, 3, 6, and 9 months. A digital photograph was taken of all the patients before the study and at each follow-up visit in order to evaluate the response to treatment. Baseline epidemiologic characteristics were analyzed, as was response at each visit, according to the decrease in color intensity and in volume. Treatment response was classified as growth, stabilization, partial response, or complete response (Table 1). Adverse effects were also recorded. All the data were collected and processed using SPSS version 17.0. Qualitative variables were expressed as absolute and relative frequency (percentage), and quantitative variables were expressed as mean (SD). The Pearson χ2 test was applied to detect a possible relationship between the response to treatment and age at initiation of treatment (less than or more than 6 months and less than or more than 12 months), as well as the association between location of the lesion and response to treatment. The differences between the duration of treatment in patients aged less than and more than 6 months and between patients aged less than and more than 12 months were analyzed using the t test with a Levene correction. The Shapiro-Wilk test was applied to determine whether the quantitative variables were normally distributed (n < 50).
Classification of Treatment Response.
| Response | Color | Volume |
| Growth | Increased intensity | Increased volume |
| Stabilization | Maintenance or clearance <25% | Maintenance or reduction <25% |
| Partial | Partial clearance | Partial volume reduction |
| Complete | Total clearance or residual telangiectasia | Complete flattening |
In cases of different responses for volume and color, the finding was classified in the next lowest category.
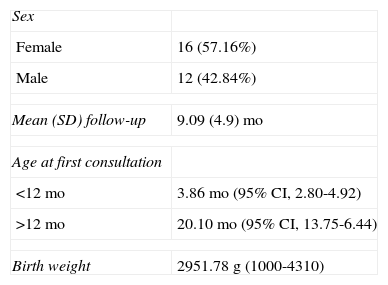
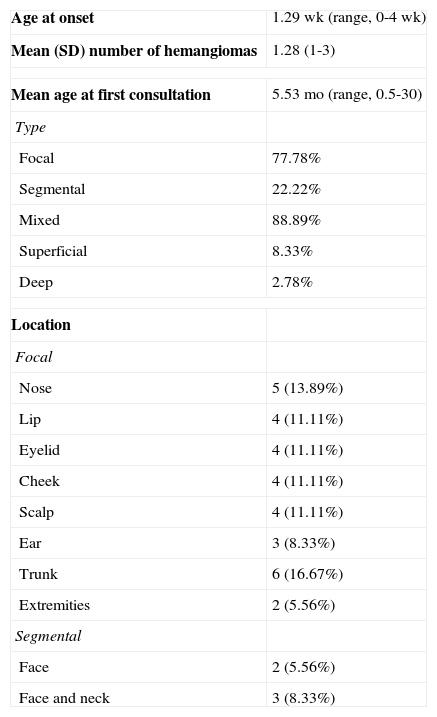
We analyzed 28 patients with 36 hemangiomas. Demographic data are shown in Table 2. Mean age at onset was 1.29 weeks and the mean number of tumors per child was 1.28. The characteristics of the tumors are shown in Table 3. Ulceration developed in 38.89% of tumors before initiation of treatment. Twenty-one patients (27 tumors) started treatment before age 12 months. The mean age at the initiation of treatment with propranolol was 3.86 months (95% confidence interval [CI], 2.80-4.92; range, 1.43-10.23) for patients aged less than 12 months and 20.10 months (95% CI, 13.75-26.44; range, 14.00-32.67) for patients aged more than 12 months. Table 3 shows the location and subtype of the hemangiomas.
Characteristics of Hemangioma of Infancy.
| Age at onset | 1.29 wk (range, 0-4 wk) |
| Mean (SD) number of hemangiomas | 1.28 (1-3) |
| Mean age at first consultation | 5.53 mo (range, 0.5-30) |
| Type | |
| Focal | 77.78% |
| Segmental | 22.22% |
| Mixed | 88.89% |
| Superficial | 8.33% |
| Deep | 2.78% |
| Location | |
| Focal | |
| Nose | 5 (13.89%) |
| Lip | 4 (11.11%) |
| Eyelid | 4 (11.11%) |
| Cheek | 4 (11.11%) |
| Scalp | 4 (11.11%) |
| Ear | 3 (8.33%) |
| Trunk | 6 (16.67%) |
| Extremities | 2 (5.56%) |
| Segmental | |
| Face | 2 (5.56%) |
| Face and neck | 3 (8.33%) |
Five patients received oral corticosteroids before initiating propranolol. In 4 cases, these drugs were withdrawn after a few weeks, with no signs of regrowth. Four patients had large segmental hemangiomas on the face and 1 had a focal lesion on the upper eyelid. In 3 of these patients (aged 1.5, 2, and 6 months), the lesion continued to grow despite treatment with oral prednisolone (3 or 5 mg/kg/d), and oral propranolol was started while the dose of prednisolone was tapered over 3 weeks. In the 2 remaining cases, including the patient with the eyelid lesion, the hemangioma decreased in size with prednisolone at 3 mg/kg/d; however, prednisolone was tapered over 3 weeks as oral propranolol began. The patients were aged 3 and 4 months when propranolol was initiated. One had a mixed segmental hemangioma on the parotid region and ear and needed simultaneous treatment for 4 months with oral corticosteroids (3 mg/kg/d) and oral propranolol (4 mg/kg/d) due to regrowth when corticosteroids were stopped or the dose of propranolol reduced. Only 1 of these patients had PHACE syndrome with persistent trigeminal artery and long segment hypoplasia (>1cm) in both vertebral arteries and the basilar artery. The segmental cervicofacial hemangioma occluded the midline of the right eye. The patient did not respond to oral prednisolone; therefore, treatment with propranolol was started and administered up to 2 mg/kg/d, with a complete response at 16 months and no adverse effects. Only 1 patient was treated using previous local infiltration of corticosteroids; no patients received vincristine or interferon.
A response to propranolol was observed in all patients at 48 hours. In all patients (both less than and more than 12 months), the color of the tumor changed from bright red to dull red, and the lesion softened. The eye opened spontaneously in all affected patients within 7 days of treatment (range, 2-7 days). In 9 cases of ulcerated hemangioma (25%), cure was complete after a mean of 61 days (range, 15-120 days). In one patient with 3 ulcerated tumors, treatment was combined with pulsed dye laser, as the ulceration was persistent.
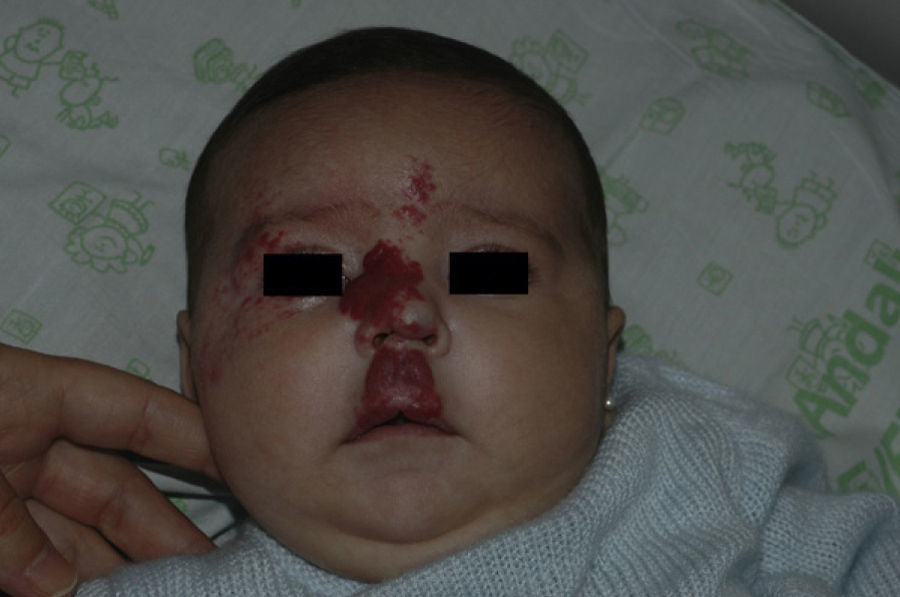
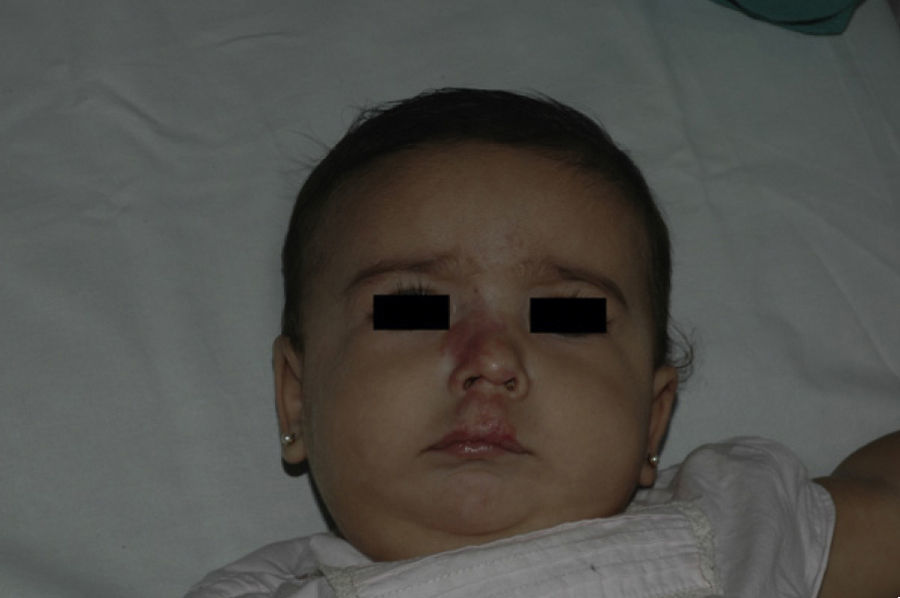
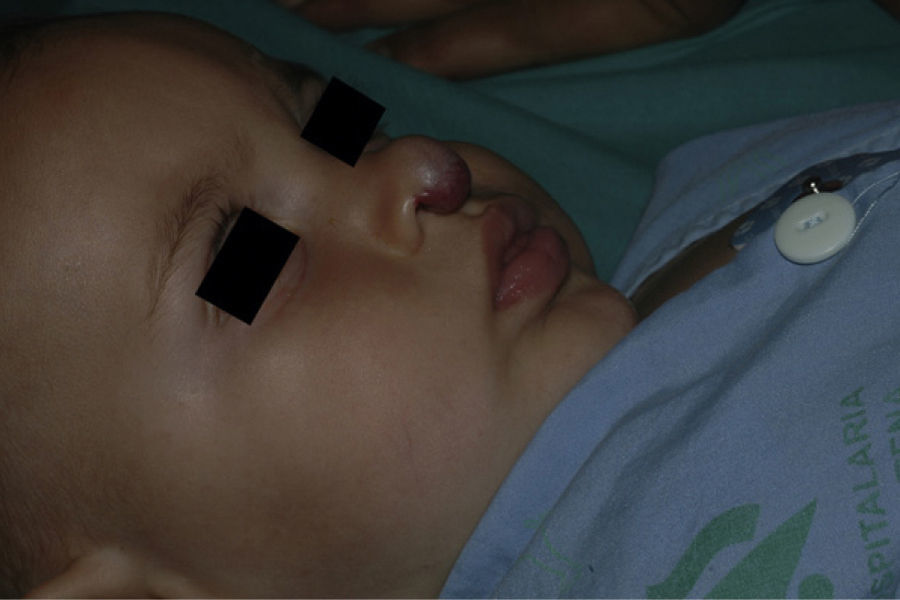
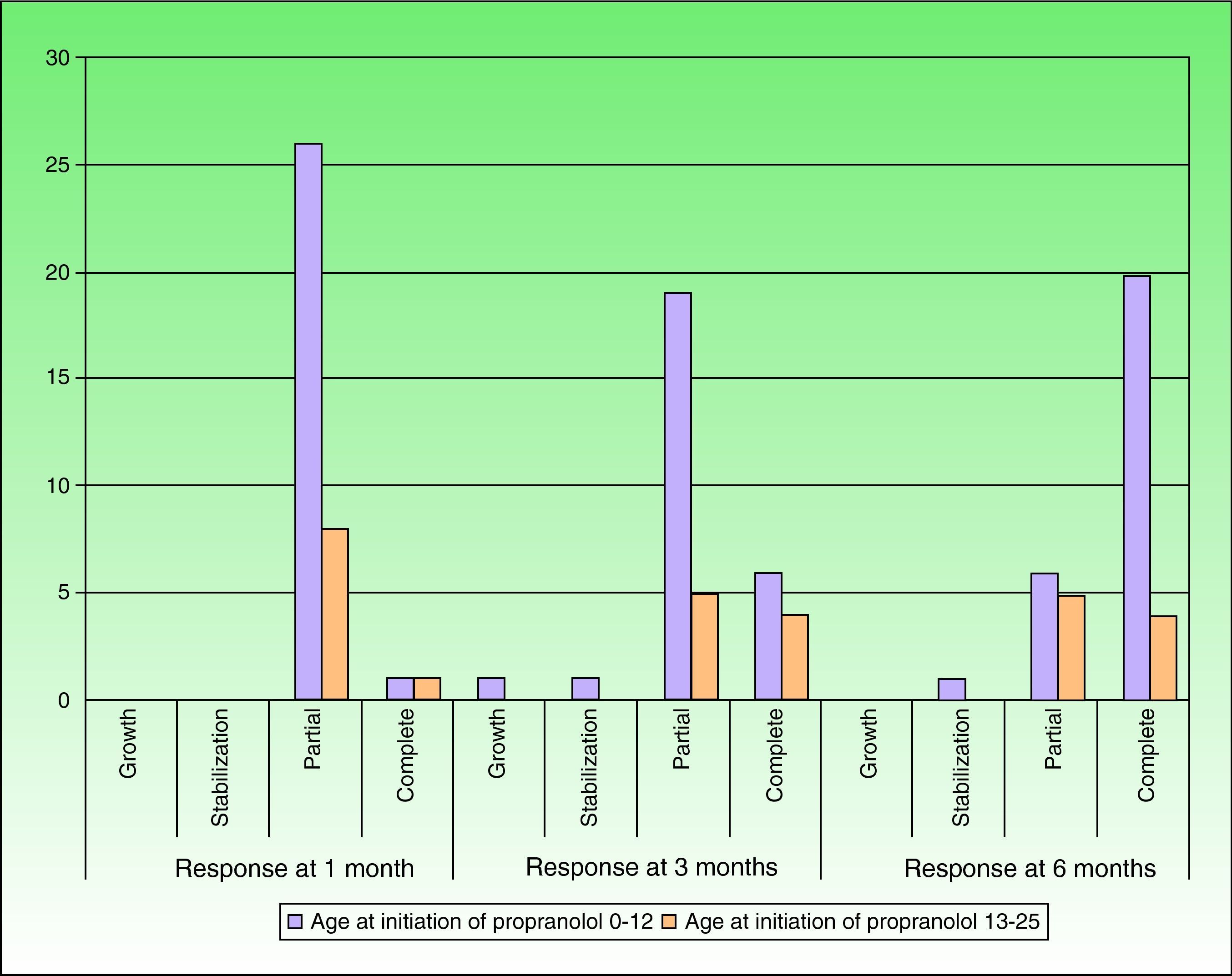
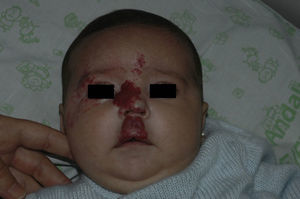
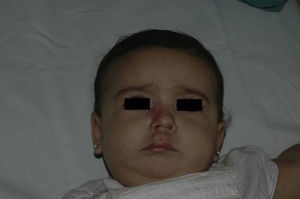
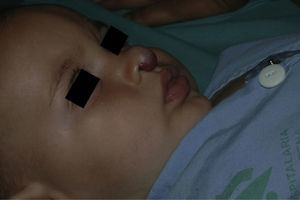
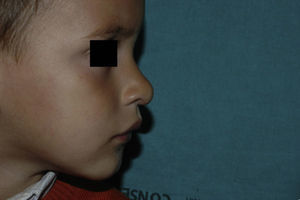
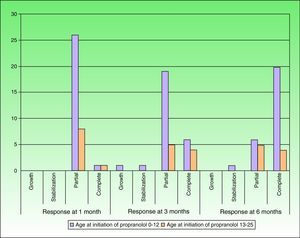
After 1 month of treatment, a complete response was observed in 6.1% of the hemangiomas and a partial response in 93.9%. At 3 months, a complete response was observed in 27.7% of patients and a partial response in 66.6%. At this point, the dose was increased in 2 patients with hemangioma to 2-4 mg/kg/d, owing to stabilization or growth of the lesion accompanied by severe deformity. At 6 months, the response was complete in 66.6% of patients and partial in 30.5%; the response was stable in 2.7% of the treated tumors (Figs. 1–4). Response by patient age (less than or more than 12 months) is shown in Figure 5. The differences in response found between patients aged less than or more than 6 months or less than or more than 12 months were not statistically significant (Pearson χ2; P=.261 and P=.432, respectively).
Analysis of the response to treatment by location showed that response was complete at 6 months in the 6 tumors affecting the eyelids (4 focal and 2 segmental) and the 4 tumors on the scalp. Of the 5 tumors on the tip of the nose, 4 had a complete response and 1 had a partial response at 6 months.
Mean duration of treatment was 8.7 months (range, 2-16 months). No statistically significant differences were observed in duration of treatment between patients aged less than or more than 6 months or between patients aged less than and more than 12 months (t test with a Levene correction, P=.341 and P=.643, respectively). In all the treated patients, the criterion for discontinuing propranolol was absence of a visible improvement for at least 1 month and absence of regrowth when the dose of propranolol was reduced to 1 mg/kg/d and after complete suspension. When the dose of propranolol was reduced from 2 to 1 mg/kg/d, it was necessary to increase the dose to 2 mg/kg/d due to regrowth in some cases (4 segmental hemangiomas in children aged less than 12 months, 2 deep focal tumors, and 1 mixed focal tumor in children aged over 12 months). After complete suspension in children aged less than 12 months, 2 tumors grew again and a further 2 had a slight increase in coloring of the residual telangiectasia that did not require treatment to be restarted. Regrowth was only observed in 1 patient from the group of children aged more than 12 months, and there was no requirement for treatment to be restarted. We did not find a statistically significant association between the location of the lesions and response to treatment (Pearson χ2, P=.412). Adverse effects were only observed in 2 patients, both of whom had an episode of hypotension during the first month of treatment (at 2 mg/kg/d). The first case (BP, 62/45 mm Hg) was asymptomatic and resolved spontaneously in 15 minutes. Treatment for regrowth was reintroduced at 1 mg/kg/d; hypotension (BP, 50/35 mm Hg) was again recorded, although it was asymptomatic and self-limiting, and treatment was discontinued. In the second case, hypotension (BP, 60/35 mm Hg) was accompanied by lethargy and the patient was seen in the emergency department of Hospital Infantil Virgen del Rocío. The condition resolved spontaneously after 30 mintues and treatment was discontinued. During treatment, 3 patients had acute bronchitis, which resolved without complications after temporary suspension of oral propranolol and treatment with inhaled salbutamol and oral corticosteroids. No regrowth of the hemangioma was observed.
Of the 6 patients with hemangiomas affecting the eyes (4 focal hemangiomas and 2 segmental tumors), the midline was affected in only 2 cases. The eyelid was completely occluded after the first month of life in 1 of these patients, although only for a short period (7 days). Anisometropia was ruled out in all cases based on refraction assessed using retinoscopy with dilated pupils. Visual acuity was assessed in children aged less than 2 years using the fix and follow reflex, which made it possible to rule out moderate and severe ambliopia. According to the judgement of the pediatric ophthalmologist, no children required treatment. Nevertheless, these patients are still being monitored by the pediatric ophthalmology unit.
DiscussionWe found treatment with propranolol to be effective in all cases, with a complete response at 6 months in 66.6%. The results were apparent during the first hours of treatment in the form of changes in the color and consistency of the lesions. This finding is consistent with those of other published series.14–19 The action of the drug was particularly striking in tumors on the eyelid, scalp, and tip of the nose. However, we did not find a statistically significant association between the location of the lesions and response to treatment, probably as a result of the small sample size. Of note, oral propranolol did not only lead to stabilization of growth—as occurs with corticosteroids—but the improvement also continued in many cases until regression was complete at very young ages, thus considerably reducing the duration of these lesions. Furthermore, this rapid and significant response was also observed in all tumors in patients aged over 12 months. With corticosteroids, on the other hand, treatment is only effective during the proliferative phase.4,19 We observed a more marked response at 6 months of treatment in patients treated before they were 12 months old (74% vs 44.6%). It is noteworthy that, in patients aged more than 12 months, both complete response and partial response were the same at 3 and 6 months of treatment, probably because, at this age, it is more difficult to obtain a complete response with no residual deformity using medical treatment alone. In cases treated early, complete remission occurred at a mean age of 8.7 months, suggesting that treatment with propranolol should be started during the proliferative phase and before most lesions reach their maximum size, at around 5 months of life.15,16,18,19 We believe that empirical treatment should be maintained in involuting lesions (late treatment) until the optimal result is obtained.
Oral propranolol should not be used as a first-line treatment option in patients with hemangioma of infancy and intracranial vascular abnormalities. However, we elected to initiate treatment with oral propranolol in the patient with PHACE syndrome included in this study, since a large segmental hemangioma completely occluded the midline of the right eye despite treatment with oral corticosteroids, and the cerebral vascular abnormalities did not affect the internal carotid artery, which has been implicated in all cases of cerebrovascular accidents associated with PHACE.20 Furthermore, that patient needed the longest course of treatment (until 18 months of age) of all those who started propranolol early (16 months of treatment).
In our experience, propranolol should be withdrawn gradually: when the dose is reduced, recoloring or regrowth can be observed in some cases, thus making it necessary to prolong treatment. By adopting this approach, we did not have to reintroduce the drug after complete withdrawal.
In the case of ulceration, the results obtained with oral propranolol to date have been variable.19,21,22 The mean healing time for ulcerated hemangiomas in our series was 61 days, and in 1 patient pharmacotherapy was combined with pulsed dye laser. We think that the presence of ulceration does not in itself indicate administration of propranolol, as in our series we observed no differences in improvement of symptoms or healing time compared with other treatments.22–30 Randomized comparative studies are necessary to clarify this point.
We found most adverse effects to be mild and predictable at the dose administered, although one of the patients who experienced hypotension required emergency care. Blood glucose was not monitored during treatment. Given that one of the possible adverse effects of oral propranolol is low blood glucose, parents were advised to administer treatment every 12 hours with meals and to withdraw treatment temporarily in case of vomiting; however, it was not necessary to stop treatment for this reason at any time during follow-up.
Our study is limited by its small sample size, which could mean that the differences detected did not reach statistical significance. Nevertheless, our sample size is similar to that of other studies, and our findings increase worldwide clinical experience with propranolol in the treatment of hemangioma of infancy.
In conclusion, according to the present study, treatment with oral propranolol solution at 2 mg/kg/d is an effective, safe, and well-tolerated option for the treatment of hemangioma of infancy. The effect is apparent during the first few hours after starting treatment, which should be maintained for several weeks, generally up to 6 months, in order to obtain a considerable improvement and halt the natural course of the lesion. This treatment seems to be more effective in lesions in the proliferative phase; however, in our experience, it does not significantly reduce symptom severity or time to scarring when ulceration is present.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Bernabeu-Wittel J, et al. Tratamiento con propanolol oral para hemangiomas infantiles graves: serie de 28 pacientes. Actas Dermosifiliogr. 2011;102:510–16.