Cetuximab and panitumumab are monoclonal antibodies that target the epidermal growth factor receptor (EGFR) in the treatment of metastatic colorectal cancer. Most patients develop a papulopustular rash, which may predict tumor response. We studied whether the other adverse cutaneous effects associated with these monoclonal antibodies are also clinical predictors of response. We also reviewed publications describing approaches to treating the papulopustular rash since no evidence-based guidelines have yet been published.
Material and methodsWe performed a retrospective study of 116 patients with metastatic colorectal cancer receiving anti-EGRF therapy with cetuximab or panitumumab at Hospital Universitario Donostia.
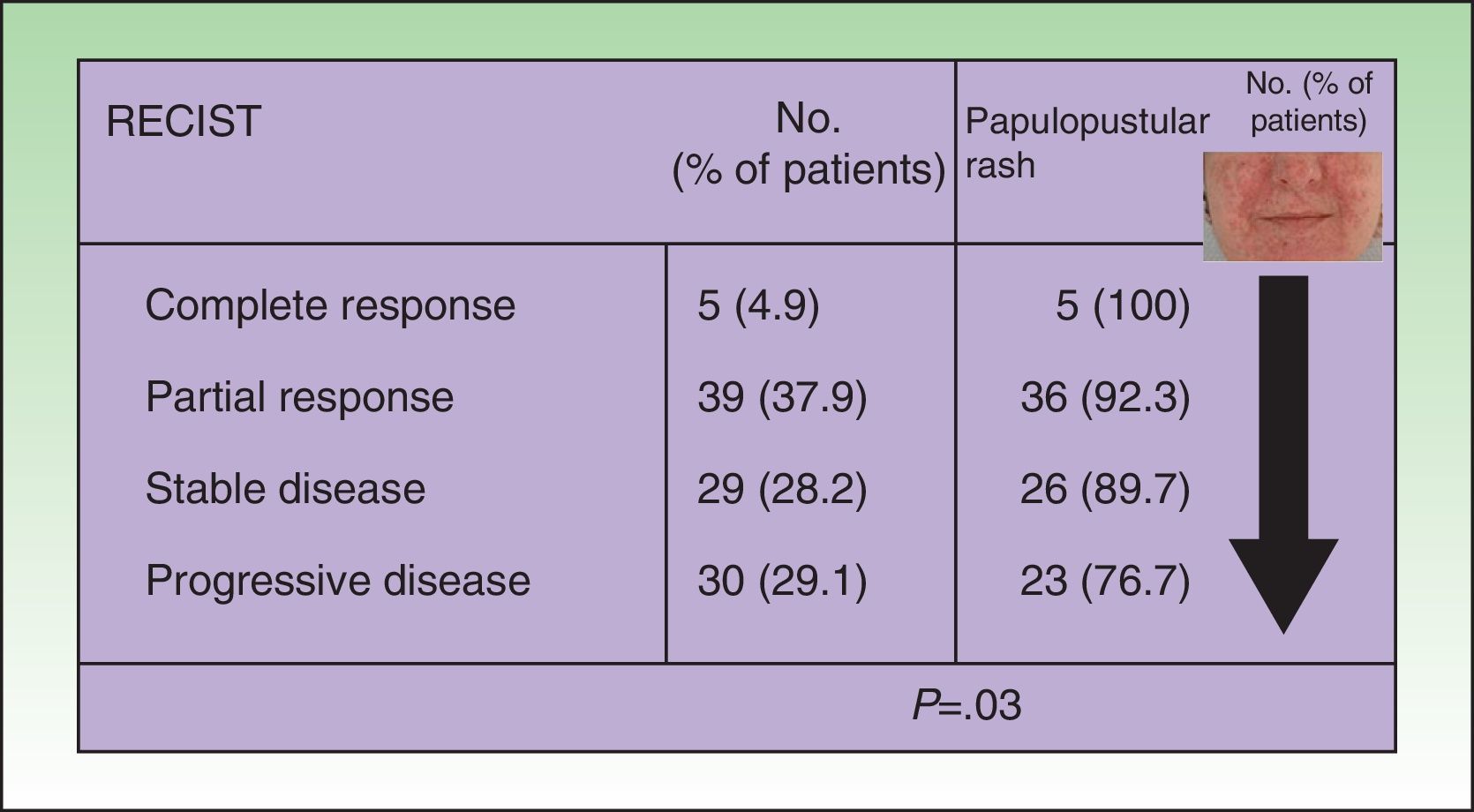
ResultsIn total, 81.9% of the patients developed a papulopustular rash. Patients who received the most cycles of treatment with the EGFR inhibitor were at the highest risk of developing the rash, and these patients also had the most severe rash reactions (P=.03). All of the patients who exhibited a complete tumor response had the rash, and the incidence of rash was lower in patients with poor tumor response (P=.03). We also observed an association between tumor response and xerosis (53.4% of the patients who developed xerosis also exhibited tumor response, P=.002). The papulopustular rash was managed according to an algorithm developed by our department.
ConclusionsSevere papulopustular rash and xerosis may be clinical predictors of good response to anti-EGFR therapy. Patients who develop a papulopustular rash should be treated promptly because suboptimal treatment of this and other adverse effects can lead to delays in taking the prescribed anti-EGFR dose or to interruption of therapy.
Cetuximab y panitumumab son anticuerpos anti-factor de crecimiento epidérmico (anti-EGFR) usados para el cáncer colorrectal metastásico. La mayoría de los pacientes desarrollan una erupción papulopustulosa que podría predecir la respuesta tumoral. Además, producen otros efectos adversos cutáneos, por lo que hemos estudiado si estos también podrían ser predictores clínicos de respuesta. Así mismo, hemos realizado una revisión del tratamiento de la erupción papulopustulosa, ya que no existen directrices basadas en la evidencia.
Material y métodosEstudio retrospectivo de 116 pacientes. Se incluyeron pacientes afectos de cáncer colorrectal metastásico en tratamiento con los anticuerpos anti-EGFR, cetuximab o panitumumab, en el Hospital Universitario Donostia.
ResultadosEl 81.9% de los pacientes desarrolló la erupción papulopustulosa, siendo el riesgo mayor y de mayor intensidad cuantos más ciclos de anti-EGFR se administraban (p=0,03). Todos los pacientes que obtuvieron una respuesta tumoral completa desarrollaron la erupción. Cuanto peor era la respuesta tumoral, menor era la frecuencia de la erupción (p=0,03). También se encontró una asociación entre la xerosis y la respuesta tumoral (el 53,4% de los que obtuvieron respuesta tumoral desarrollaron xerosis, p=0,002). El manejo de la erupción papulopustulosa se llevó a cabo mediante un algoritmo desarrollado por nuestro servicio.
ConclusionesEn la práctica clínica la erupción papulopustulosa grave y la xerosis pueden ser predictores clínicos de buena respuesta al tratamiento anti-EGFR. Los pacientes con esta erupción deben tratarse precozmente, ya que el tratamiento subóptimo de estos efectos secundarios puede conllevar un retraso en la dosis o su interrupción.
Epidermal growth factor receptor inhibitors (EGFR/HER1/Erb1) are antitumor agents that have emerged in recent years to treat advanced-stage solid tumors.1,2 Inhibition of EGFR-mediated signals is achieved with 1) monoclonal antibodies, namely cetuximab (Erbitux) and panitumumab (Vectibix), which are used mainly in metastatic colorectal cancer,1,3 but also in squamous cell carcinoma of the head and neck and skin2,4 and 2) low-molecular-weight drugs, namely erlotinib (Tarceva), gefitinib (Iressa), used in lung cancer and pancreatic cancer, and lapatinib (Tykerb), used in breast cancer.5 These drugs prevent adenosine triphosphate binding to the intracellular portion of EGFR. Unlike conventional chemotherapy, EGFR inhibitors are used at an optimal biological dose to achieve better tolerance.
EGFR is a transmembrane glycoprotein expressed in the skin and in the epithelial cells of 30% to 100% of solid tumors.2 The activation of EGFR by its growth factor ligands triggers a series of processes that regulate epidermal proliferation, differentiation, apoptosis, migration and synthesis of inflammatory cytokines.6 EGFR therefore plays a role in the development and normal differentiation of epidermal keratinocytes, stimulating their growth, protecting against UV-induced damage, inhibiting inflammation, and favoring wound healing. By altering keratinocyte proliferation and differentiation, EGFR inhibition exerts an antineoplastic effect, but it also triggers a series of cutaneous toxic effects, such as abnormal follicular keratinization and a secondary inflammatory response.5,6 All EGFR inhibitors cause dose-dependent cutaneous toxicity.7
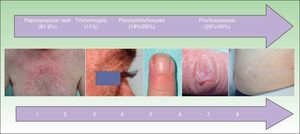
Adverse cutaneous effects are observed in sites expressing high levels of EGFR, and the most common effect is a papulopustular rash, which occurs in over 80% of patients.7,8 The rash tends to be mild, but it can be moderate or severe in up to 50% to 60% of cases,9,10 requiring dose reductions or discontinuation of antitumor treatment. Other adverse effects are paronychia, hair alterations, pruritus, and xerosis.
Research is underway to study markers that help to predict the effectiveness of EGFR inhibitors and avoid unnecessary toxicity by limiting administration to patients who will potentially benefit from them. Activating KRAS and NRAS mutations are currently the only predictors of resistance to approved anti-EGFR inhibitors used in clinical practice, and their evaluation has been included in the summary of product characteristics of cetuximab and panitumumab by the European Medicines Agency.1
Other potential clinical and genetic markers of tumor response, however, have also been investigated. Genetic studies have shown that EGFR polymorphisms involved in gene regulation are associated with rash and tumor response, although no consensus has yet been reached on their predictive value.1,3,8,11 Our group recently suggested that the single nucleotide polymorphism-216 might be a useful predictor of tumor response in metastatic colorectal cancer.12 One of the main clinical markers of response to anti-EGFR monoclonal antibodies is the development of a papulopustular skin rash.3
The existence of clinical predictors for this skin rash, however, is less clear, although young age and male sex have been proposed as the main predictors.11,13
Material and MethodsWe performed a retrospective observational study of 116 patients with metastatic colorectal cancer under treatment with intravenous anti-EGFR monoclonal antibodies (cetuximab every week or panitumumab every 2 weeks) at Hospital Universitario Donostia between January 2010 and January 2013.
The following outcome variables were analyzed: papulopustular rash, face eczema, xerosis, pruritus, paronychia, fissures, hypertrichosis, trichomegaly, sores, herpes, and inflammation of sun-damaged skin. Skin rash severity was measured using the National Cancer Institute's modified Common Terminology Criteria for Adverse Events,2,14 which is the only standardized scale currently available. The independent variables analyzed were tumor response, age, sex, place of origin, type of anti-EGFR monoclonal antibody, associated chemotherapy, culture testing, discontinuation of antitumor treatment, and treatment with tetracyclines or isotretinoin. Tumor response was assessed using the Response Evaluation Criteria in Solid Tumors.12,14
Four patients were excluded from the analysis: 3 because they died of cancer before being evaluated by the dermatology department and 1 because cetuximab treatment was discontinued following an infusion reaction. Tumor response was classified as inevaluable in 13 patients and was therefore analyzed in 103 of the 116 patients studied.
Statistical AnalysisThe association between the study variables and the presence or absence of a papulopustular rash and the severity of this rash was analyzed using the Fisher test in the case of dichotomous variables and the χ2 test in the case of variables with more than 2 categories. The association between rash and age was analyzed using the t test. Time of rash onset was analyzed by Kaplan-Meier curves, and cases in which the rash did not develop within a month of treatment initiation were treated as censored data.
The same tests were used to analyze the relationship between other toxic effects and tumor response, sex, age, associated chemotherapy, interruption of antitumor treatment, and type of EGFR inhibitor. The time it took for the papulopustular rash to appear according to the EGFR inhibitor used was compared with the log-rank test. An ordinal logistic regression model was used to investigate associations with rash severity. Variables with a P value of 0.2 or less were included in the model.
All the tests were 2-tailed and the level of significance was set at a P level of .05. The analyses were performed using SPSS v.21.
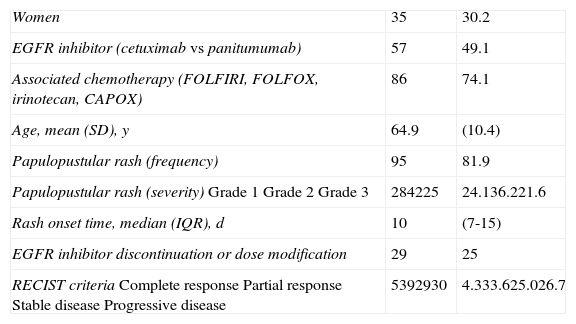
ResultsWe studied 116 patients (35 women and 81 men) aged between 31 and 86 years (mean, 64.9 years). The main epidemiological and clinical characteristics are shown in Table 1.
Sociodemographic and Clinical Characteristics of Patients.a
| Women | 35 | 30.2 |
| EGFR inhibitor (cetuximab vs panitumumab) | 57 | 49.1 |
| Associated chemotherapy (FOLFIRI, FOLFOX, irinotecan, CAPOX) | 86 | 74.1 |
| Age, mean (SD), y | 64.9 | (10.4) |
| Papulopustular rash (frequency) | 95 | 81.9 |
| Papulopustular rash (severity)Grade 1Grade 2Grade 3 | 284225 | 24.136.221.6 |
| Rash onset time, median (IQR), d | 10 | (7-15) |
| EGFR inhibitor discontinuation or dose modification | 29 | 25 |
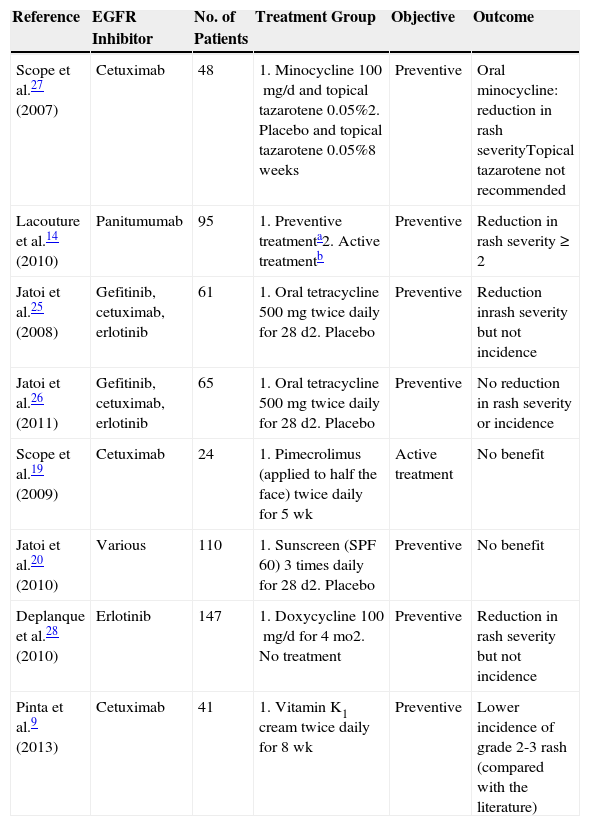
| RECIST criteriaComplete responsePartial responseStable diseaseProgressive disease | 5392930 | 4.333.625.026.7 |
Abbreviations: CAPOX, capecitabine and oxaliplatin; FOLFIRI, folinic acid (leucovorin), 5-fluorouracil, and irinotecan; FOLFOX, folinic acid (leucovorin), 5-fluorouracil, and oxaliplatin; IQR, interquartile range; RECIST, Response Evaluation Criteria in Solid Tumors.
Ninety-five patients (81.9%) developed a papulopustular rash, which was classified as moderate to severe (grades 2-3) in 70.5% of cases.
The median time between initiation of anti-EGFR therapy and the appearance of the rash was 10 days (interquartile range, 7-15 days). No significant differences were observed for time of rash onset between cetuximab and panitumumab (median, 8 vs 10 days).
Culture was performed for 22 samples. Just 2 of these were positive for Staphylococcus aureus, supporting previous reports that these pustules are sterile. Skin biopsy was performed in 2 patients and revealed neutrophilic folliculitis in both cases.
Koebner phenomenon was observed in hospitalized patients who developed the rash on pressure areas and in patients with a reservoir or an ostomy.
In the univariate analysis, papulopustular rash (presence vs absence) was a predictor of tumor response, with incidence increasing with better tumor response. All the patients who achieved complete tumor response developed a rash, and the presence of the rash became less common with poorer tumor response (P=.03, Fig. 1). Although the association lost strength when the rash was classified by severity, it maintained a clearly linear trend (P=.05, Table 2).
Association between tumor response and development of papulopustular rash. The incidence of rash was greater in patients who responded to anti-epidermal growth factor receptor (EGFR) therapy. All the patients who achieved complete tumor response developed the rash, but this became less common with worsening tumor response. Just 76.7% of patients with progressive disease developed a papulopustular rash (P=.03, χ2 test). RECIST indicates Response Evaluation Criteria in Solid Tumors.
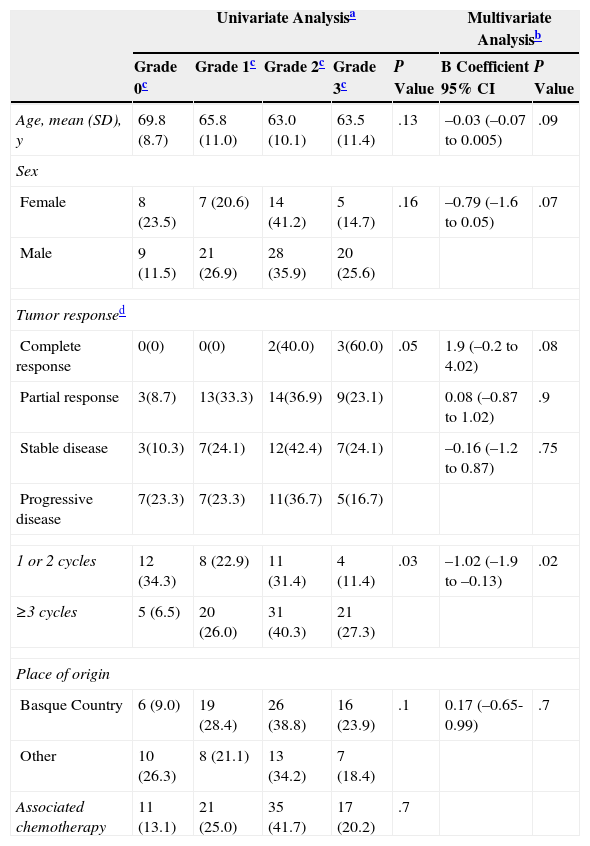
Predictors of Frequency and Severity of Papulopustular Rash.
| Univariate Analysisa | Multivariate Analysisb | ||||||
|---|---|---|---|---|---|---|---|
| Grade 0c | Grade 1c | Grade 2c | Grade 3c | P Value | B Coefficient 95% CI | P Value | |
| Age, mean (SD), y | 69.8 (8.7) | 65.8 (11.0) | 63.0 (10.1) | 63.5 (11.4) | .13 | –0.03 (–0.07 to 0.005) | .09 |
| Sex | |||||||
| Female | 8 (23.5) | 7 (20.6) | 14 (41.2) | 5 (14.7) | .16 | –0.79 (–1.6 to 0.05) | .07 |
| Male | 9 (11.5) | 21 (26.9) | 28 (35.9) | 20 (25.6) | |||
| Tumor responsed | |||||||
| Complete response | 0(0) | 0(0) | 2(40.0) | 3(60.0) | .05 | 1.9 (–0.2 to 4.02) | .08 |
| Partial response | 3(8.7) | 13(33.3) | 14(36.9) | 9(23.1) | 0.08 (–0.87 to 1.02) | .9 | |
| Stable disease | 3(10.3) | 7(24.1) | 12(42.4) | 7(24.1) | –0.16 (–1.2 to 0.87) | .75 | |
| Progressive disease | 7(23.3) | 7(23.3) | 11(36.7) | 5(16.7) | |||
| 1 or 2 cycles | 12 (34.3) | 8 (22.9) | 11 (31.4) | 4 (11.4) | .03 | –1.02 (–1.9 to –0.13) | .02 |
| ≥3 cycles | 5 (6.5) | 20 (26.0) | 31 (40.3) | 21 (27.3) | |||
| Place of origin | |||||||
| Basque Country | 6 (9.0) | 19 (28.4) | 26 (38.8) | 16 (23.9) | .1 | 0.17 (–0.65-0.99) | .7 |
| Other | 10 (26.3) | 8 (21.1) | 13 (34.2) | 7 (18.4) | |||
| Associated chemotherapy | 11 (13.1) | 21 (25.0) | 35 (41.7) | 17 (20.2) | .7 | ||
Multivariate ordinal logistic regression analysis was used to investigate the capacity of the clinical variables analyzed to predict the development of a papulopustular rash (Table 2). In the univariate analysis, only number of treatment cycles and tumor response were significantly associated with the development of a skin rash. Number of cycles retained its significance in the multivariate analysis, with a higher number of cycles associated with a greater risk of more severe rash (P=.02). Severe rash was also associated with complete tumor response (P=.08), male sex (P=.07), and younger age (P=.09).
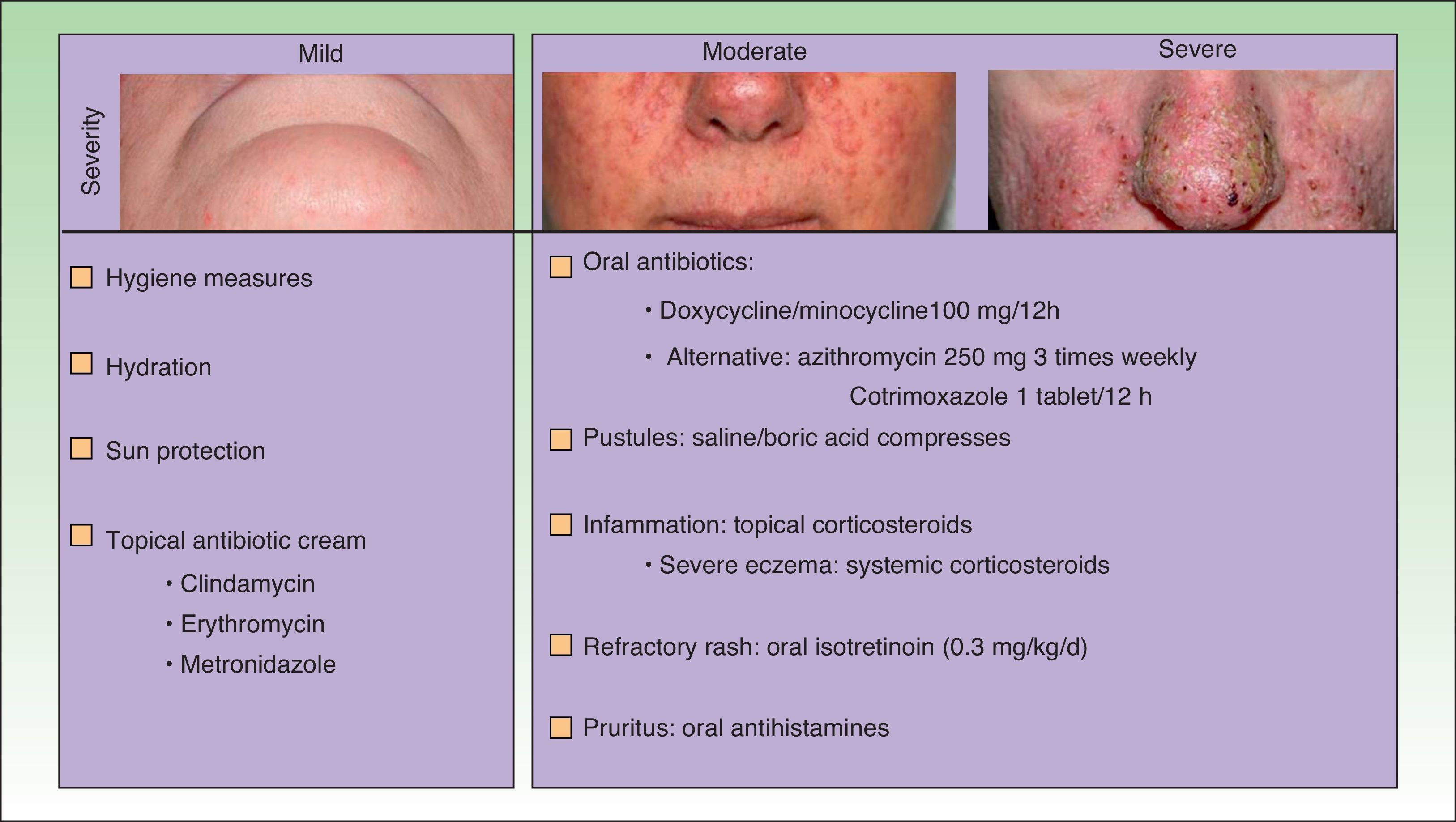
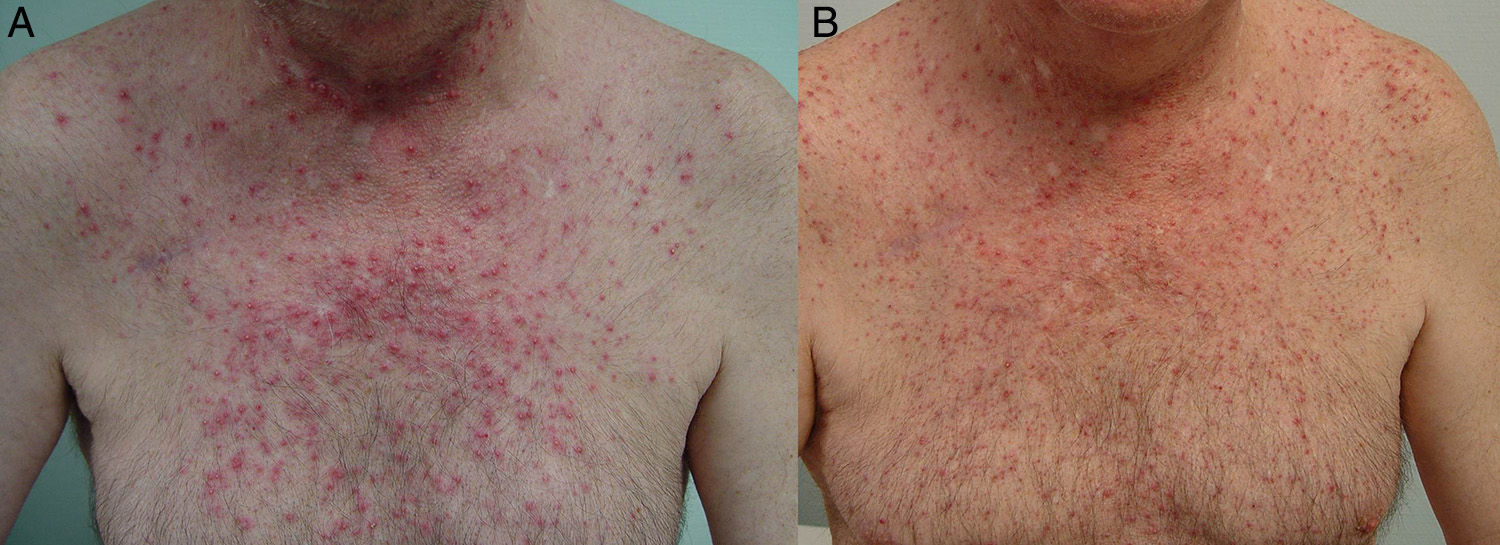
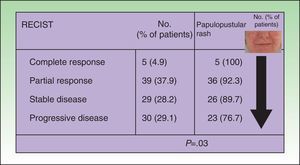
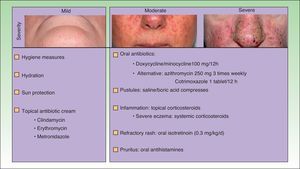
The papulopustular rash was treated as soon as possible to avoid having to discontinue anti-EGFR therapy due to toxic effects. Fig. 2 shows the treatment algorithm used at our department to treat papulopustular rash according to severity. All patients with a grade 2 or 3 rash were treated with oral tetracyclines (Fig. 3A), which achieved adequate control (Fig. 3B). Isotretinoin was needed to treat 3 patients with a more refractory skin rash, and a good response was obtained in 2 of these. The third patient did not tolerate this treatment, leading to discontinuation of anti-EGFR therapy.
In the current series, neither oral tetracyclines nor isotretinoin altered tumor response.
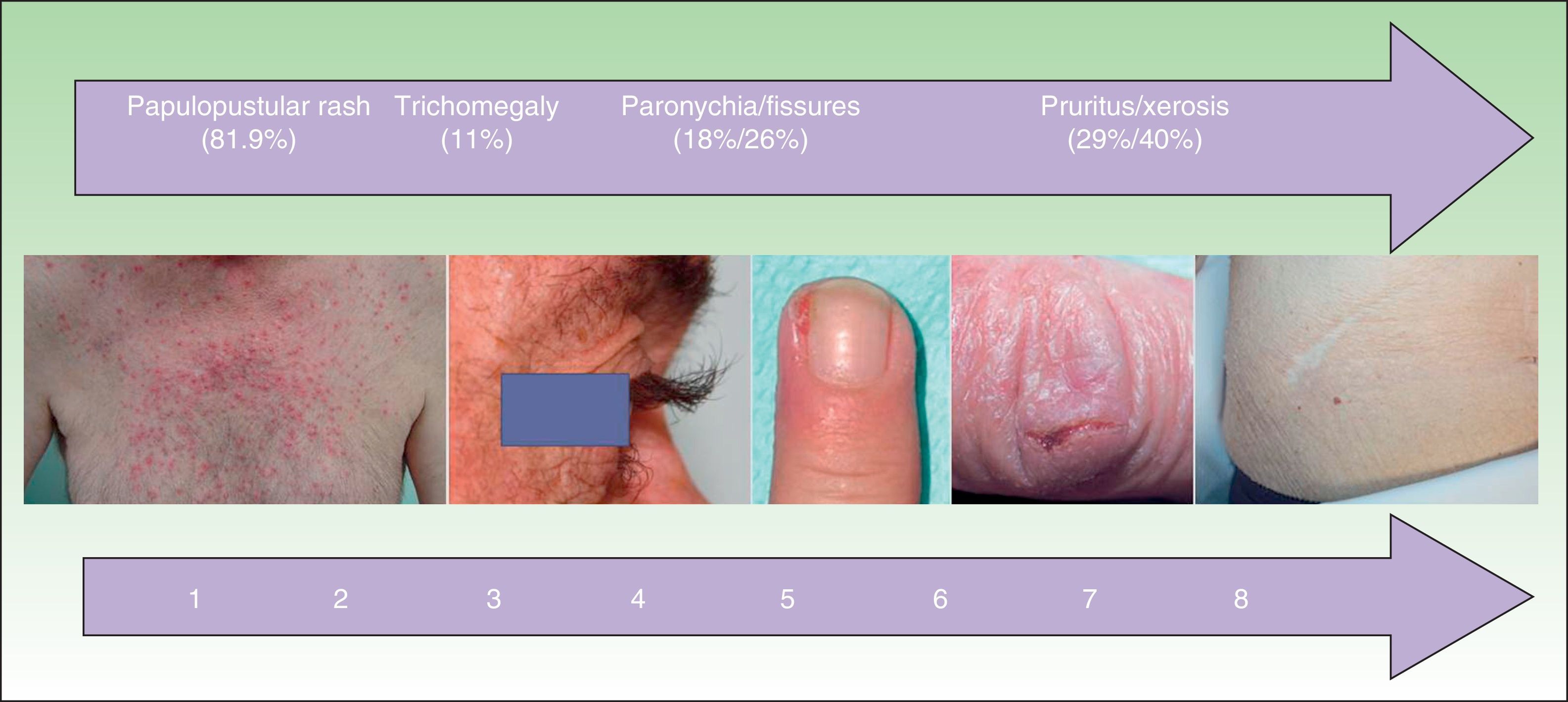
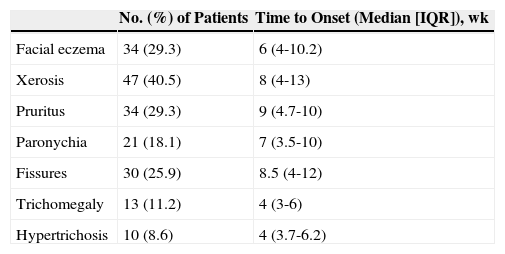
Other Cutaneous ToxicitiesThe epidemiological characteristics of cutaneous toxicities other than papulopustular rash are shown in Table 3 and Fig. 4. We analyzed the relationship between these adverse cutaneous effects and sex, age, EGFR inhibitor, associated chemotherapy, and tumor response.
Frequency and Time to Onset of Other Cutaneous Toxicities.
| No. (%) of Patients | Time to Onset (Median [IQR]), wk | |
|---|---|---|
| Facial eczema | 34 (29.3) | 6 (4-10.2) |
| Xerosis | 47 (40.5) | 8 (4-13) |
| Pruritus | 34 (29.3) | 9 (4.7-10) |
| Paronychia | 21 (18.1) | 7 (3.5-10) |
| Fissures | 30 (25.9) | 8.5 (4-12) |
| Trichomegaly | 13 (11.2) | 4 (3-6) |
| Hypertrichosis | 10 (8.6) | 4 (3.7-6.2) |
Abbreviation: IQR, interquartile range.
Facial eczema was more common in patients treated with panitumumab than with cetuximab (23 vs 11, P=.02) and in some cases it was so severe it needed treatment with systemic corticosteroids.
Xerosis was more common in patients who responded to antitumor therapy and in elderly patients, although the difference did not reach statistical significance in the second group of patients. Thirty-nine (53.4%) of the 73 patients who achieved tumor response developed xerosis, compared with just 6 (20.0%) of those who did not (P=.002). The xerosis had an ichthyosis-like appearance in 6 cases. Pathologic examination was performed in 1 of the patients and showed lesions consistent with acquired ichthyosis.
No significant associations were found for any of the other cutaneous toxicities.
Cultures were ordered for 5 of the 21 paronychias to rule out secondary infection. Two were positive for S aureus, 1 was positive for Streptococcus pyogenes, and 1 was positive for Candida albicans.
The most common hair alterations were trichomegaly, hypertrichosis, and hirsutism (Table 3), although there were also 5 cases of diffuse hair loss (1 woman and 4 men).
Mucosal involvement was observed in 16 patients. Eleven developed herpes lesions on the oral and/or genital mucosa, but 10 of these were also receiving chemotherapy. Five of the patients undergoing chemotherapy had oral sores, and 2 of these needed treatment with systemic corticosteroids to control the sores.
Photosensitivity occurred in patients receiving chemotherapy with 5-fluorouracil derivatives. However, 3 patients being treated with panitumumab monotherapy experienced inflammation of existing sun-damaged skin. This association has been described very rarely with EGRF inhibitors.
It was possible to discontinue methotrexate therapy in 1 patient with psoriasis and psoriatic arthritis whose psoriasis improved during anti-EGFR therapy.
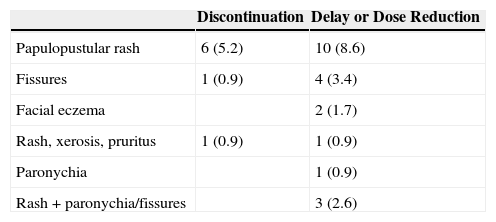
Finally, anti-EGFR therapy had to be discontinued in 8 patients (6.8%) due to cutaneous toxicity and to be delayed or reduced in an additional 21 patients (18.1%) (Table 4). The main reasons were papulopustular rash, fissures, and paronychia.
Anti-Epidermal Growth Factor Therapy Discontinuation, Delay, or Dose Reduction.a
| Discontinuation | Delay or Dose Reduction | |
|---|---|---|
| Papulopustular rash | 6 (5.2) | 10 (8.6) |
| Fissures | 1 (0.9) | 4 (3.4) |
| Facial eczema | 2 (1.7) | |
| Rash, xerosis, pruritus | 1 (0.9) | 1 (0.9) |
| Paronychia | 1 (0.9) | |
| Rash + paronychia/fissures | 3 (2.6) |
Although papulopustular rash can be considered an in vivo marker of anti-EGFR activity, the relationship between other EGFR inhibitor–induced cutaneous toxicities and tumor response has not been described in the literature.3,8 In the current series, we observed an association between xerosis and anti-EGFR therapy, with higher rates seen in patients who responded better to treatment. This is the first time this association has been described and we believe that, like papulopustular rash, xerosis may be a clinical marker of tumor response.
Our findings also confirm the previously described association between papulopustular rash and tumor response,3,12 with patients who responded better to anti-EGFR therapy showing an increased risk of severe rash. These results add strength to the idea that tumor response and cutaneous toxicity have a similar mechanism of action. Patients who develop more severe skin rash would appear to benefit more from anti-EGFR therapy. Several authors have suggested employing a dose escalation to rash strategy in patients who do not develop the papulopustular rash. This strategy involves administering increased doses of the drug until the patient develops the rash.15 Other authors, in turn, have suggested that the absence of the rash after a given time should prompt the discontinuation of treatment, but this is not a decisive factor in current clinical practice.1 In our opinion, and considering that a small subgroup of patients who do not develop a rash might actually benefit from anti-EGFR therapy, this decision should be based on a series of clinical and genetic markers that would permit more individualized therapy.
On investigating the association between the development of a papulopustular rash and other clinical predictors, we found a trend towards significance for age and sex, supporting findings by Jatoi et al.11 These authors reported that the rash was more common in male patients and younger patients, and hypothesized that older patients have lower levels of EGFR and that both androgens and estrogens interact with this receptor.
Numerous treatment options have been described over the years for cutaneous toxicity induced by EGFR inhibitors, but no consensus has yet been reached. Given the current lack of evidence-based guidelines, current treatment strategies are based on expert opinions10 and clinical experience.
Several topical treatments have been used, but without proven success,9,16–18 and just 3 randomized trials have been conducted. Scope et al.19 evaluated the use of topical pimecrolimus applied to half of the face, but found no clinical benefit, like Jatoi et al.,20 who evaluated the use of preventive treatment with a sunscreen for a month compared with placebo. Finally, Pinta et al.9 found that the application of vitamin K1 cream appeared to have a beneficial effect, but their comparative data were from reports in the literature (Table 5).
Randomized Clinical Trials of EGFR Inhibitor–Induced Papulopustular Rash.
| Reference | EGFR Inhibitor | No. of Patients | Treatment Group | Objective | Outcome |
|---|---|---|---|---|---|
| Scope et al.27 (2007) | Cetuximab | 48 | 1. Minocycline 100mg/d and topical tazarotene 0.05%2. Placebo and topical tazarotene 0.05%8 weeks | Preventive | Oral minocycline: reduction in rash severityTopical tazarotene not recommended |
| Lacouture et al.14 (2010) | Panitumumab | 95 | 1. Preventive treatmenta2. Active treatmentb | Preventive | Reduction in rash severity ≥2 |
| Jatoi et al.25 (2008) | Gefitinib, cetuximab, erlotinib | 61 | 1. Oral tetracycline 500mg twice daily for 28 d2. Placebo | Preventive | Reduction inrash severity but not incidence |
| Jatoi et al.26 (2011) | Gefitinib, cetuximab, erlotinib | 65 | 1. Oral tetracycline 500mg twice daily for 28 d2. Placebo | Preventive | No reduction in rash severity or incidence |
| Scope et al.19 (2009) | Cetuximab | 24 | 1. Pimecrolimus (applied to half the face) twice daily for 5 wk | Active treatment | No benefit |
| Jatoi et al.20 (2010) | Various | 110 | 1. Sunscreen (SPF 60) 3 times daily for 28 d2. Placebo | Preventive | No benefit |
| Deplanque et al.28 (2010) | Erlotinib | 147 | 1. Doxycycline 100mg/d for 4 mo2. No treatment | Preventive | Reduction in rash severity but not incidence |
| Pinta et al.9 (2013) | Cetuximab | 41 | 1. Vitamin K1 cream twice daily for 8 wk | Preventive | Lower incidence of grade 2-3 rash (compared with the literature) |
Abbreviations: EGFR, epidermal growth factor receptor; SPF, sun protection factor.
Considering other papulopustular conditions, such as rosacea and acne, oral tetracyclines, which have both anti-inflammatory and antiangiogenic properties—described by Fernandez-Guarino et al. in 20062,21—would be the first-line treatment for papulopustular rash. Favorable outcomes have been reported for azithromycin,22 isotretinoin,23 and acitretin.24 Isotretinoin is recommended for more refractory cases, as it is not clear how this drug might affect the mechanism of action of EGFR inhibitors. Accordingly, some authors have recommended limiting its use in this setting.7Table 5 summarizes the findings of 5 randomized clinical trials that have analyzed the prophylactic use of oral tetracyclines in papulopustular rash.14,25–28
Lacouture et al.14 compared the prophylactic and active use of doxycycline to improve panitumumab-induced cutaneous toxicity and found a 50% reduction in the incidence of moderate to severe rash (Table 5).
Jatoi et al., Scope et al., and Deplanque et al. compared, respectively, the use of oral tetracyclines, minocycline, and doxycycline with placebo from the start of anti-EGFR therapy, and found that these drugs decreased the severity and number of lesions, but not the incidence of the rash.26–28 In a more recent report, Jatoi et al. concluded that tetracyclines were not an effective prophylactic treatment for papulopustular rash,26,28 and Scope et al.27reported no additional benefits after 8 weeks of treatment (Table 5). Given the controversy surrounding the effectiveness of tetracyclines, which was highlighted in a recent meta-analysis,28 these drugs cannot be recommended as preventive standard therapy in EGRF inhibitor–induced rash. Nevertheless, according to the American clinical practice guidelines for the prevention and treatment of EGFR inhibitor–associated dermatologic toxicities doxycycline 100mg should be administered every 12 to 24hours for the first 6 weeks of treatment.10 The treatment algorithm used in our hospital establishes tetracyclines as a first-line treatment for papulopustular rash, as in our experience they achieve good control. We usually maintain the treatment for the duration of anti-EGFR therapy, or for less time if the rash is controlled sooner. No specific studies have analyzed treatments for other cutaneous toxicities induced by EGFR inhibitors, although doxycycline has been proposed as a potential option for paronychia. Management strategies typically include hygiene measures, emollients, and topical antiseptics, antibiotics, and corticosteroids.10 The use of sun protection measures is also important, as some patients experience inflammation of existing sun damage, possibly due to the high levels of EGFR that tend to be found in the follicular ostium and in precursor skin cancer lesions.29
Finally, although there have been reports of worsening psoriasis,30 1 patient in our series experienced improvement of his psoriasis during anti-EGFR therapy, possibly due to the antiproliferative effect of EGFR inhibition, as hyperproliferation has been implicated in the pathogenesis of this skin condition.7
ConclusionsThe results of our study suggest that, like papulopustular rash, xerosis may be a predictor of tumor response in patients treated with EGFR inhibitors. We therefore believe that both markers should be used to identify patients who might benefit from anti-EGFR therapy.
Furthermore, treatment of papulopustular rash should be initiated early and continued, as suboptimal treatment can lead to treatment delays or interruption in patients who are benefiting from anti-EGFR therapy.
In our experience, treatment should be individualized and adjusted over time according to the course of the different cutaneous adverse effects.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Jaka A, Gutiérrez-Rivera A, López-Pestaña A, del Alcázar E, Zubizarreta J, Vildosola S, et al. Factores predictores de respuesta y revisión de la toxicidad cutánea de cetuximab y panitumumab en 116 pacientes. Actas Dermosifiliogr. 2015;106:483–492.