Phototherapy is a treatment option for atopic dermatitis recommended by several guidelines.
ObjectiveTo perform a systematic review of the efficacy of different modalities of phototherapy and photochemotherapy in moderate to severe atopic dermatitis.
Material and methodsWe considered all randomized clinical trials (RCTs) performed in patients with atopic dermatitis, and accepted all outcome measures. Articles were identified via an online search of the MEDLINE (via Ovid) and Embase databases and the Cochrane Central Register of Controlled Trials. We also searched for clinical trials registered in Current Controlled Trials and in the World Health Organization's International Clinical Trials Registry Platform.
ResultsTwenty-one RCTs (961 patients) were included in the qualitative analysis. Two of the trials included children and adolescents (32 patients). The efficacy of narrow-band UV-B and UV-A1 phototherapy was similar for the different outcome measures contemplated. Two RCTs assessed the efficacy of psoralen plus UV-A therapy (PUVA). No serious adverse events were described. In general, the publications reviewed were characterized by a high risk of bias and poor reporting of methodology and results.
ConclusionsThere is evidence for the use of narrow-band UV-B and UV-A1 phototherapy in moderate to severe atopic dermatitis. Evidence supporting the use of PUVA in atopic dermatitis is scarce and there is little information on the use of phototherapy in childhood. For the purpose of future studies, it would be advisable to use comparable criteria and scales for the evaluation of disease severity and patients, to standardize radiation methods, and to establish a minimum follow-up time.
La fototerapia es una opción terapéutica empleada en dermatitis atópica (DA) y recomendada en múltiples guías.
ObjetivosEvaluar la eficacia de las distintas modalidades de fototerapia y fotoquimioterapia en el tratamiento de pacientes con DA moderada-grave, mediante una revisión sistemática.
Material y métodosConsideramos los ensayos clínicos aleatorizados (ECA) realizados en pacientes con DA, aceptando cualquier medida de desenlace. Localizamos los artículos mediante una búsqueda electrónica, utilizando Medline (vía Ovid), Embase y Cochrane Central Register of Controlled Trials. Adicionalmente, buscamos los ensayos clínicos registrados en Current Controlled Trials y en la WHO International Clinical Trials Registry Platform.
ResultadosIncluimos 21 ECA en el análisis cualitativo (961 pacientes). Dos ECA incluyeron niños y adolescentes (32 pacientes). Las modalidades UVBBE y UVA1 mostraron resultados de eficacia similares en diversas medidas de desenlace. Dos ECA incluyeron la terapia PUVA. No se describieron efectos secundarios graves. En general, el riesgo de sesgos fue elevado y la calidad de las publicaciones baja, en cuanto a comunicación de la metodología empleada y los resultados obtenidos.
ConclusionesExiste evidencia para el uso de UVBBE y UVA1 en DA moderada-grave. La evidencia para el uso de PUVA en DA es mínima, así como los datos del uso de la fototerapia en la infancia. En futuros estudios sería recomendable estandarizar los criterios de gravedad de la DA y las escalas de valoración de los pacientes, homogeneizar las técnicas de irradiación y establecer un periodo de seguimiento mínimo.
Atopic dermatitis (AD) is a chronic and recurring inflammatory disease that affects individuals of any age, especially children and young adults.1
Therapeutic guidelines encourage an individualized approach to treating AD and include recommendations on skin moisturizing, corticosteroids and/or topical calcineurin inhibitors, systemic antihistamines, and topical or systemic antibiotics, when required. The most complex cases often require treatment with photochemotherapy, systemic corticosteroids, and/or immunosuppressants, sometimes in combination.1,2
Phototherapy has been widely used in AD since the 1970s. Over the years, various modalities of phototherapy with UV light have been introduced, namely psoralen plus UV-A (PUVA or photochemotherapy), broadband UV-B (BB UV-B, 280-315nm), narrowband UV-B (NB UV-B, peak=311nm), UV-A (315-400nm), UV-A1 (340-400nm), and UV-AB (UV-A followed by UV-B or simultaneous exposure to both). Data from controlled clinical trials on the efficacy of phototherapy are scarce.
The first systematic review3 on phototherapy in the management of AD was published in 2007. Another recent systematic review4 provided—like the present study—an update on the results of clinical trials published in the past few years, including studies on PUVA.
The main objective of this study was to evaluate, through a systematic review of the literature, the efficacy of the various modalities and regimens of phototherapy and photochemotherapy used in the treatment of patients with moderate to severe AD.
Material and MethodsIdentification of StudiesWe used the MEDLINE (via Ovid) and Embase databases and the Cochrane Central Register of Controlled Trials (CENTRAL) to identify articles through the seventh week of 2013 (Embase) and through February 18, 2013 (MEDLINE and CENTRAL). The search terms related to patient type and interventions were Medical Subject Headings (MeSH) terms and free terms. The search terms related to design type were based on the Cochrane Highly Sensitive Search Strategy.5 The results were limited to studies conducted in humans and published in English, French, or Spanish. Before carrying out the online searches, we identified 2 articles that met the inclusion criteria and were expected to be retrieved by the searches in all of the databases.6,7 The search strategies will be provided on request by the corresponding author.
Additionally, we carried out a search in PubMed Clinical Queries using the narrow search filter and a manual search of potentially relevant references cited in the included studies. We also asked Dr José Manuel Carrascosa, an expert on the treatment of AD with phototherapy, to review our results in order to detect the possible absence of relevant studies.
Finally, we also searched for clinical trials registered with Current Controlled Trials (www.controlled-trials.com) and with the World Health Organization's International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/).
Study Inclusion CriteriaWe considered all randomized clinical trials (RCTs) performed in patients clinically diagnosed with atopic dermatitis, without any age limit. We accepted as interventions all types of phototherapy as well as phototherapy in combination with psoralens (photochemotherapy). We excluded animal studies, studies in which the intervention was applied in localized areas (hands or feet), and studies in which the use of topical and/or systemic corticosteroids and immunosuppressants was not systematized or controlled. We accepted all outcome measures, although measures of disease improvement or quality of life were preferred over economic or laboratory measures.
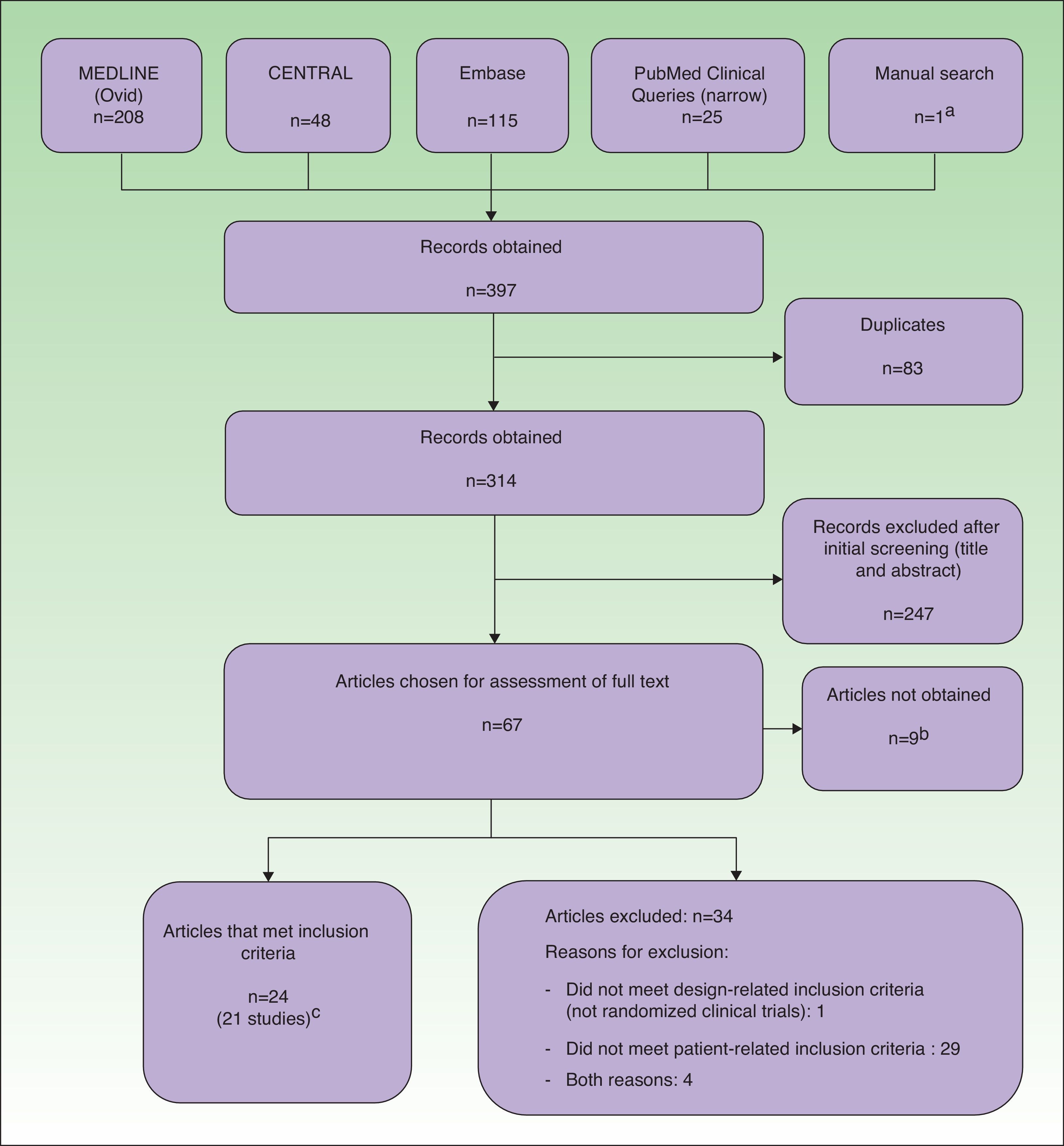
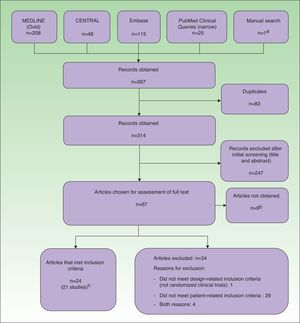
Data Management and ExtractionThe results obtained by the searches were downloaded to a reference management software package (EndNote, Thomson Reuters, 2011), which allowed us to filter the articles by title and abstract. Potentially relevant articles were independently evaluated in their entirety by pairs of reviewers. During data extraction, we used a questionnaire that included a study quality assessment form based on the Cochrane Collaboration's risk of bias tool.5 Throughout the process, all duplicate, rejected, and selected references were recorded in a PRISMA flow diagram (Fig. 1).
ResultsExcluded StudiesThirty-four articles were excluded from the analysis because they had a non-RCT design and/or they did not meet the patient-related inclusion criteria (Fig. 1).
Included StudiesTwenty-four records corresponding to 21 RCTs (961 patients) were included in the analysis. Three of the studies8–10 were also published as conference proceedings.11–13 Two studies14,15 included children and adolescents (32 patients). Two studies14,16 were carried out in Asia and the rest were carried out in Europe. Three were multicenter studies.6,17,18 Most of the studies compared different types of phototherapy, including high-dose (HD) UV-A1, medium-dose (MD) UV-A1, UV-B, UV-A and UV-B combination therapy (UV-AB), NB UV-B, and PUVA. Other modalities were excimer laser (EL),8 full-spectrum-light phototherapy (FSL),16 and synchronous balneophototherapy (sBPT).6 One study evaluated the utility of a skin-reflectance-guided UV-B regimen as a means of reducing the cumulative dose of radiation.19 Three studies compared phototherapy with other treatments, namely ciclosporin,17 topical pimecrolimus,14 and topical corticosteroid therapy combined with phototherapy.15 The shortest follow-up period was 4 weeks9,14,20 and the longest periods were 6 months6,21 and 12 months.7,17 Several studies had no follow-up period.10,15,18,19,22–27 In most studies, the outcome measures were changes in scores on clinical scales. The most frequently used scales were SCORing Atopic Dermatitis (SCORAD) or a modified form of SCORAD,6,7,9,16,17,19,21,28 Costa's Simple Scoring System (SSS),10,15,18,27 the Leicester scale,15,27 and the severity score29 of Hanifin and Rajka.23–26 Some studies also used quality-of-life scales (Eczema Disability Index,17 Sickness Impact Profile6) and assessments of pruritus.8,14,20,22,27 Cumulative dosage was an outcome measure in 1 study19 and length of remission was used in 2 studies.7,21
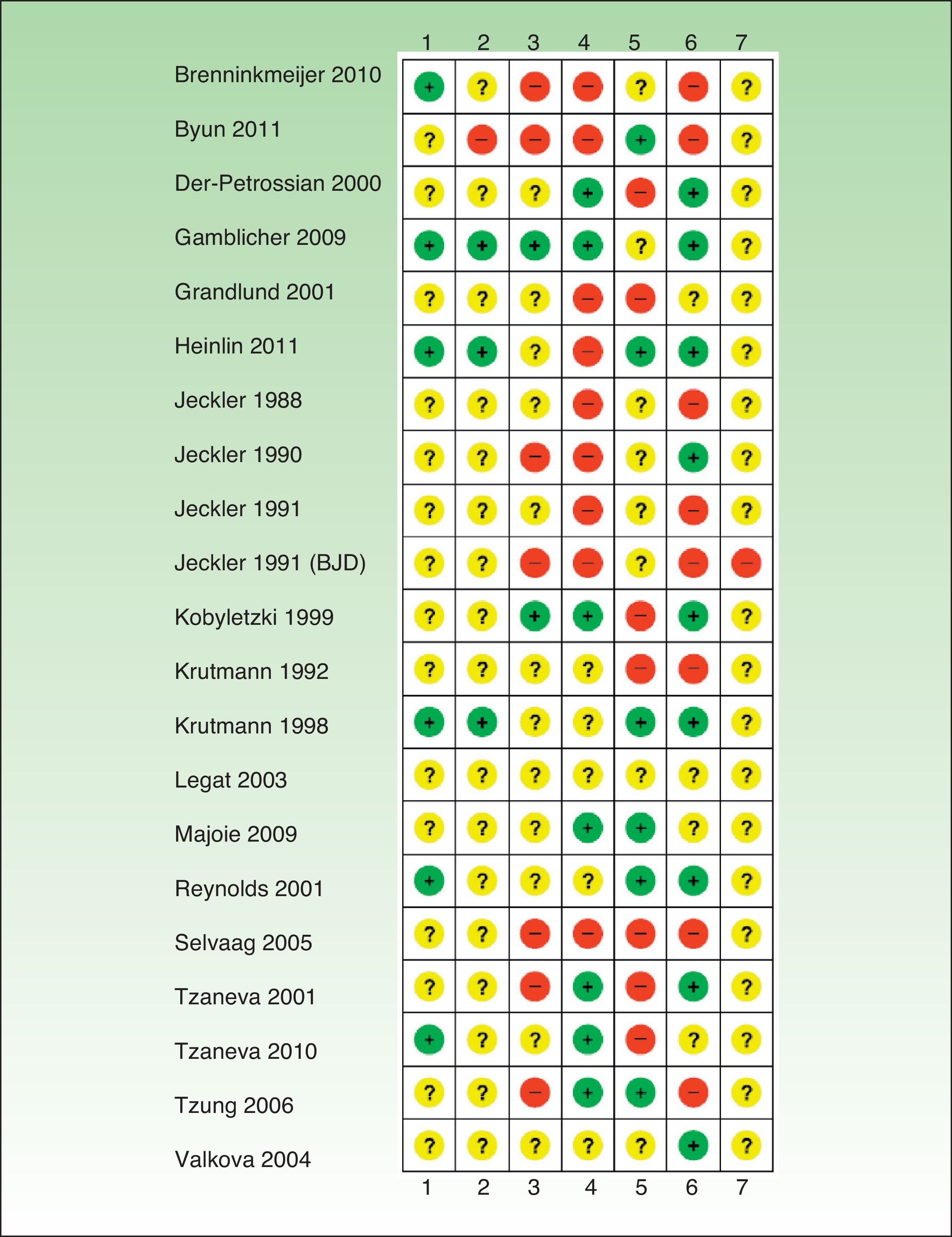
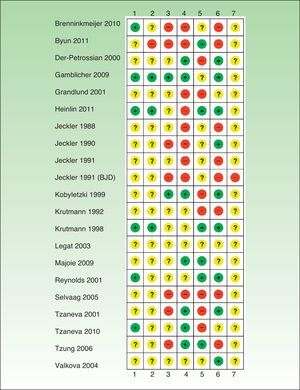
Risk of Bias in the Included StudiesIn general, the studies reviewed had a high risk of bias and relevant information was frequently missing (Tables 1–6, Fig. 2).
Studies Comparing UV-A, UV-B, and UV-AB.
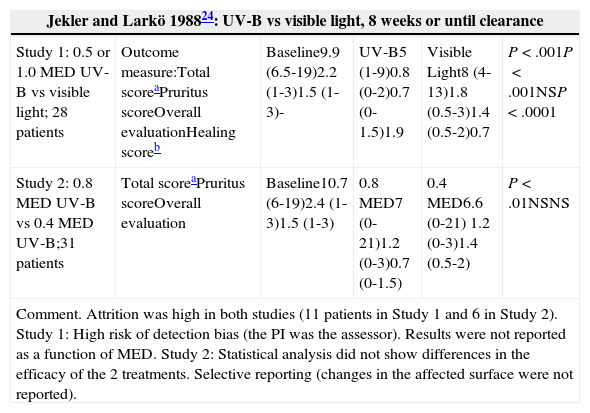
| Jekler and Larkö 198824: UV-B vs visible light, 8 weeks or until clearance | |||||
|---|---|---|---|---|---|
| Study 1: 0.5 or 1.0 MED UV-B vs visible light; 28 patients | Outcome measure:Total scoreaPruritus scoreOverall evaluationHealing scoreb | Baseline9.9 (6.5-19)2.2 (1-3)1.5 (1-3)- | UV-B5 (1-9)0.8 (0-2)0.7 (0-1.5)1.9 | Visible Light8 (4-13)1.8 (0.5-3)1.4 (0.5-2)0.7 | P<.001P<.001NSP<.0001 |
| Study 2: 0.8 MED UV-B vs 0.4 MED UV-B;31 patients | Total scoreaPruritus scoreOverall evaluation | Baseline10.7 (6-19)2.4 (1-3)1.5 (1-3) | 0.8 MED7 (0-21)1.2 (0-3)0.7 (0-1.5) | 0.4 MED6.6 (0-21) 1.2 (0-3)1.4 (0.5-2) | P<.01NSNS |
| Comment. Attrition was high in both studies (11 patients in Study 1 and 6 in Study 2). Study 1: High risk of detection bias (the PI was the assessor). Results were not reported as a function of MED. Study 2: Statistical analysis did not show differences in the efficacy of the 2 treatments. Selective reporting (changes in the affected surface were not reported). | |||||
| Jekler and Larkö 199025: UV-B vs UV-AB, 3 sessions/week for 8 weeks; 39 patients | |||||
|---|---|---|---|---|---|
| Outcome measure:Total scoreaPruritus scoreOverall evaluation | Baseline10.8 (7-19)2.4 (1-3)1.7 (1-3) | UV-B6.1 (0-17)1.2 (0-3)0.80 (0-3) | UV-AB5.2 (0-15) 1.0 (0-3) 0.65 (0-2) | P=.002P=.04P=.03 | |
| Healing scoreb (UV-B vs UV-AB, No. of patients out of 30)Subjective assessment of efficacyGreater use of hydrocortisoneAdverse effects | 3 points, 4 vs 6; 2 points, 21 vs 20; 1 point, 4 vs 3; 0 points, 0 vs 0; –1 points, 1 vs 1.UV-AB preferred by 14; both preferred equally by 10.UV-B side, 3/20; UV-AB side, 1/20; same on both sides, 16/20.Mild or moderate xerosis, 15 vs 13; severe xerosis, 5 vs 2; mild or moderate burns, 15 vs 3; severe burns, 6 vs 0. | ||||
| Comment. Combined UV-AB therapy may be superior to UV-B with this regimen. Risk of bias: randomization process not described; high risk of detection bias in participants, researchers, and outcome assessor. | |||||
| Jekler and Larkö 199126: UV-A vs UV-B, 3 sessions/week for 8 weeks or until healing; 33 patients | |||||
|---|---|---|---|---|---|
| Outcome measure:Total scoreaPruritus scoreOverall evaluation | Baseline10.3 (6-18)2.2 (1-3)1.8 (1-3) | UV-A5.5 (1-12)(0-2)1 (0-2) | UV-B6.4 (3-15.5)1.3 (0-2)1.3 (0.5-2.5) | P<.02P=.01 | |
| Healing scoreb (UV-A vs UV-B)ExtentSubjective assessment of efficacy | 3 points, 1 vs 0; 2 points, 14 vs 13; 1 point, 6 vs 6; 0 points, 0 vs 2; –1 points, 0 vs 0.From 10.1% to 5.4% (UV-A) and from 10% to 6.2% (UV-B) (P<.05).13 noticed improvement in the area treated with UV-A; 4 noticed no difference. | ||||
| Comment. UV-A may be superior to UV-B with this regimen. Randomization not described. High risk of performance bias, detection bias, and selective reporting: Only some variables were analyzed; 4 patients did not complete the subjective assessment of the efficacy of the interventions. | |||||
| Jekler and Larkö 199123 | |||||
|---|---|---|---|---|---|
| Study 1:Low-dose UV-B vs UV-AB;20 patients | Outcome MeasureTotal scoreaPruritus scoreOverall evaluation | Baseline10.8 (7-15.5)2.4 (1-3)1.9 (1-2.5) | UV-B8.8 (4.5-14) 1.5 (0-2)1.8 (1-2.5) | UV-AB5.3 (1.1-11) 0.8 (0-2)0.9 (0-2) | P<.001P<.001P<.001 |
| Healing scoreb (patients in UV-B group vs patients in UV-AB group): 3 points, 0 vs 2; 2 points, 5 vs 15; 1 point, 11 vs 1; 0 points, 2 vs 0; –1 points, 0 vs 0. | |||||
| Study 2:UV-A vs UV-AB; 28 patients | UV-AB yielded better results than UV-A in total score and overall evaluation. No differences in pruritus. | ||||
| Comment. UV-AB was apparently superior to UV-B and UV-A. High risk of detection bias (blinding of outcome assessor), attrition bias (no ITT analysis performed in excluded individuals, lack of patient questionnaires), and reporting bias (more variables were recorded than were analyzed; specific results of Study 2 were not reported). | |||||
Abbreviations: ITT, intention to treat; MED, minimal erythema dose; NS, not specified; PI, principal investigator; UV-AB; combined UV-A and UV-B phototherapy.
Studies Comparing HD UV-A1 (130J/cm2) and MD UV-A1.
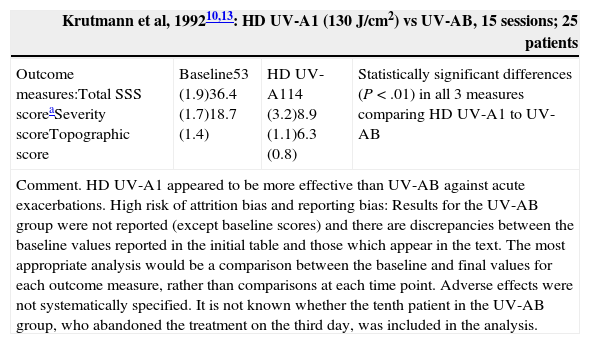
| Krutmann et al, 199210,13: HD UV-A1 (130J/cm2) vs UV-AB, 15 sessions; 25 patients | |||
|---|---|---|---|
| Outcome measures:Total SSS scoreaSeverity scoreTopographic score | Baseline53 (1.9)36.4 (1.7)18.7 (1.4) | HD UV-A114 (3.2)8.9 (1.1)6.3 (0.8) | Statistically significant differences (P<.01) in all 3 measures comparing HD UV-A1 to UV-AB |
| Comment. HD UV-A1 appeared to be more effective than UV-AB against acute exacerbations. High risk of attrition bias and reporting bias: Results for the UV-AB group were not reported (except baseline scores) and there are discrepancies between the baseline values reported in the initial table and those which appear in the text. The most appropriate analysis would be a comparison between the baseline and final values for each outcome measure, rather than comparisons at each time point. Adverse effects were not systematically specified. It is not known whether the tenth patient in the UV-AB group, who abandoned the treatment on the third day, was included in the analysis. | |||
| Krutmann et al, 199818: HD UV-A1 vs UV-AB vs 0.5% fluocortolone cream or ointment, 10 days; 53 patients | |||
|---|---|---|---|
| Outcome measure: SSS score | Significant differences were found in SSS scores in favor of HD UV-A1 and fluocortolone as compared to UV-AB (P<.0001 in both cases) and in favor of HD UV-A1 as compared to fluocortolone (P<.002). | ||
| Comment. HD UV-A1 appeared to be superior to UV-AB and at least as effective as topical treatment with fluocortolone. The randomization sequence was explicitly described; block randomization was used. High risk of attrition bias: Results were not specified; only the statistical significance levels of the differences obtained were reported and represented in a figure. The most appropriate analysis would be a comparison between the baseline and final values for each outcome measure, rather than comparisons at each time point. No adverse effects were described. | |||
| Tzaneva et al, 200121: HD UV-A1 (130J/cm2 or a dose equivalent to 1 MED if <130J/cm2, with increments of 10J/cm2 up to a maximum of 130J/cm2) vs MD UV-A1 (50% of the HD UV-A1 regimen), 5 sessions/week for 3 weeks and 6 months of follow-up | |||
|---|---|---|---|
| Outcome measures:Modified SCORAD,b week 1Modified SCORAD, week 2Modified SCORAD, week 3 | HD UV-A133.4% (8.8%-52.7%)38.4% (1.5%-56.7%)34.7% (0%-46.9%) | MD UV-A129.7% (8.3%-46.8%)36.4% (12.6%-56.6%)28.2% (0%-46.9%) | P>.05 in all cases |
| Recurrences: 6 patients at 2, 4, and 12 weeks; 1 patient in remission at 6 months.There were no bilateral differences in time until recurrence or in intensity of recurrence. | |||
| Comment. There were no statistically significant differences in the efficacy of the 2 treatments, although the sample size was small. Of the 10 initial patients, only 7 were followed up and recurrences were frequent. Risk of bias related to randomization (the procedure is not described). Patients were not masked. “Recurrences” were not defined. | |||
| Kobyletzki et al, 1999,9 Kobyletzki, 199912: Cold-light UV-A1 vs UV-A1 vs UV-AB, 5 sessions/week for 3 weeks and 4 weeks of follow-up | |||
| Outcome measures:CR90% clearance60% clearanceBaseline SCORADcSCORAD week 3SCORAD week 7 | Cold-light UV-A185.4%27.1%58.3%71.7 (12.6)23.3 (10.6) (P<.05)24.9 (10.2) | UV-A177.3%15.9%61.4%69.8 (10.2)28.8 (6.9) (P<.05)30.8 (9.2) | UV-AB37.5%6.3%31.3%71 (9.4)41.4 (9.9) (P<.05)52.3 (11.4) |
| Attrition: 5 patients in the UV-A1 group (discomfort, pruritus, and exacerbation). | |||
| Comment. Cold-light UV-A1 appeared to be superior to UV-A1 and both appeared to be superior to UV-AB, although the decrease in SCORAD scores was significant in all 3 modalities. After 4 weeks of follow-up, SCORAD scores remained lowest with cold-light UV-A1. The randomization method was not described. No ITT analysis was performed and attrition was high in some groups. | |||
Abbreviations: CR, complete remission; HD, high-dose; ITT, intention to treat; MD, medium-dose; MED, minimal erythema dose; SCORAD, SCORing Atopic Dermatitis; SSS, Simple Scoring System; UV-AB; combined UV-A and UV-B phototherapy.
Costa's Simple Scoring System scores 10 severity criteria (erythema, edema, vesicles, oozing, crusts, lichenification, desquamation, pruritus, and sleep loss) on a scale of 0 to 6 and 10 topographic sites (face, neck, anterior trunk, posterior trunk, buttocks, arms, hands, legs, knees, and feet) on a scale of 0 to 3, according to the extent of the lesions.
Studies Comparing NB UV-B and MD UV-A1.
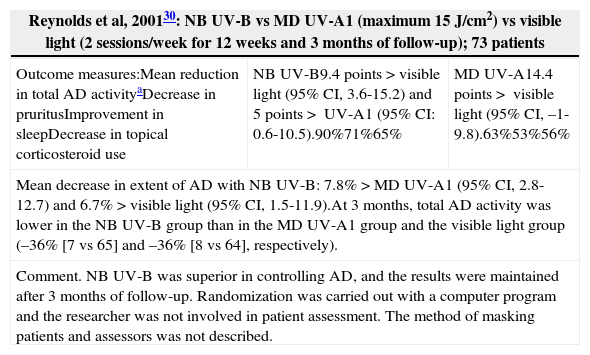
| Reynolds et al, 200130: NB UV-B vs MD UV-A1 (maximum 15J/cm2) vs visible light (2 sessions/week for 12 weeks and 3 months of follow-up); 73 patients | ||
|---|---|---|
| Outcome measures:Mean reduction in total AD activityaDecrease in pruritusImprovement in sleepDecrease in topical corticosteroid use | NB UV-B9.4 points>visible light (95% CI, 3.6-15.2) and 5 points> UV-A1 (95% CI: 0.6-10.5).90%71%65% | MD UV-A14.4 points> visible light (95% CI, –1-9.8).63%53%56% |
| Mean decrease in extent of AD with NB UV-B: 7.8%>MD UV-A1 (95% CI, 2.8-12.7) and 6.7%>visible light (95% CI, 1.5-11.9).At 3 months, total AD activity was lower in the NB UV-B group than in the MD UV-A1 group and the visible light group (–36% [7 vs 65] and –36% [8 vs 64], respectively). | ||
| Comment. NB UV-B was superior in controlling AD, and the results were maintained after 3 months of follow-up. Randomization was carried out with a computer program and the researcher was not involved in patient assessment. The method of masking patients and assessors was not described. | ||
| Legat et al, 200327: NB UV-B vs MD UV-A1 (maximum dose 50J/cm2), 2 sessions/week for 8 weeks; 9 patients | ||
|---|---|---|
| Outcome measures:Decrease in SSS score,b %Decrease in Leicester score,c %Decrease in skin lesion VAS score, %Decrease in pruritus VAS score, %Overall evaluation of effect of treatment | NB UV-B40 (2-61) (P=.004)50 (2-74) (P=.01)71 (3-98) (P=.004)67 (−8-98) (P=.055)6.4 (1.2-9.2) | MD UV-A133 (8-48) (P=.055)30 (9-63) (P=.1)40 (7-99) (P=.04)34 (9-97) (P=.15)4.5 (0.5-9.1) |
| Attrition: 2 patients withdrew, after 4 and 6 weeks, respectively, because their scores after NB UV-B were<30% of the scores after MD UV-A1. | ||
| Comment. NB UV-B was more effective than MD UV-A1, and the differences in the SSS scores, the Leicester scores, and the lesion assessment VAS scores were statistically significant. No long-term follow-up was conducted. Possible performance bias and detection bias (in randomization and masking). It is not known whether the 2 patients who withdrew were included in the analysis. | ||
| Majoie et al, 200920: NB UV-B vs MD UV-A1 (30J/cm2 up to a maximum of 45J/cm2), 3 sessions/week for 8 weeks and 4 weeks of follow-up; 13 patients | |||
|---|---|---|---|
| Outcome measures:Decrease in Leicester score, medianDecrease in pruritus VAS score, median | NB UV-BFrom 18 to 10From 7 to 1.8 | MD UV-A1From 19 to 12From 7 to 4.1 | (P<.01)(P<.01) |
| There was a significant decrease in immunohistochemical findings suggestive of inflammation, and there were no significant differences between groups. | |||
| Comment. MD UV-A1 and NB UV-B appear to be effective in clinical control and control of inflammatory markers in moderate to severe AD. The results obtained after follow-up were not analyzed because of the uncontrolled use of topical corticosteroids. Unknown risk of performance bias (randomization method not described). No adverse effects were described. | |||
| Gamblicher et al, 200922: NB UV-B vs MD UV-A1 (50J/cm2) crossover study: 3 sessions/week for 6weeks →minimum 8 week washout period →3 sessions/week for 6 weeks; 47 patients | |||
|---|---|---|---|
| No interaction was found between treatment sequence and treatment effects (P=.81). | |||
| Outcome measures:SASSAD score, mean (SD) RR, %Pruritus VAS score, mean (SD) RR, %Skindex-29 score, mean (SD) RR, % | NB UV-B39.4 (24.1)25.2 (30.5)16.5 (17.6) | MD UV-A143.7 (31.4)16 (61.8)12.7 (18.8) | P=.4P=.49P=.35 |
| Adverse effects: mild erythema in 1 patient in the MD UV-A1 group and 3 patients in the NB UV-B group. | |||
| Comment. The number of patients lost to follow-up was high but balanced between the 2 groups. The follow-up period was very short. Low risk of bias: Randomization was computer-generated and concealed, masking was carried out, adverse effects were recorded, protocol was registered, and all expected outcomes were reported. | |||
Abbreviations: AD, atopic dermatitis; NB, narrowband; MD, medium-dose; RR, relative reduction; SASSAD, Six Area, Six Sign Atopic Dermatitis; SSS, Simple Scoring System; VAS, visual analog scale.
After 24 treatments. Disease activity was measured using the modified scale described by Sowden et al.40
Studies Comparing PUVA to Other Interventions.
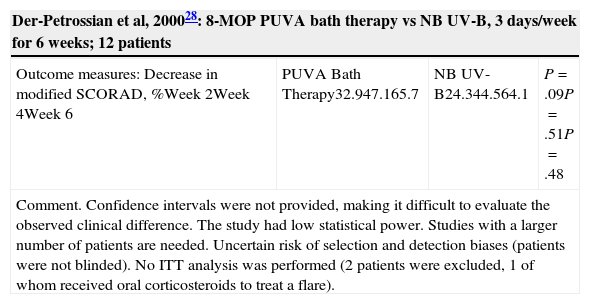
| Der-Petrossian et al, 200028: 8-MOP PUVA bath therapy vs NB UV-B, 3 days/week for 6 weeks; 12 patients | |||
|---|---|---|---|
| Outcome measures: Decrease in modified SCORAD, %Week 2Week 4Week 6 | PUVA Bath Therapy32.947.165.7 | NB UV-B24.344.564.1 | P=.09P=.51P=.48 |
| Comment. Confidence intervals were not provided, making it difficult to evaluate the observed clinical difference. The study had low statistical power. Studies with a larger number of patients are needed. Uncertain risk of selection and detection biases (patients were not blinded). No ITT analysis was performed (2 patients were excluded, 1 of whom received oral corticosteroids to treat a flare). | |||
| Tzaneva et al, 20107: MD UV-A1 (5 days/weekfor3 weeks, 70J/cm2) vs PUVA (5-MOP 1.2mg/kg; 3 days/weekfor5 weeks); 40 patients | |||
|---|---|---|---|
| Outcome measures:Length of remission, mean, wkReduction in SCORAD since baseline visit, mean (SD), % | MD UV-A1437.7 (22.8) | PUVA1254.3 (25.7) | P=.012P=.041 |
| Adverse effects: Mild palmoplantar erythema (MD UV-A1, 2; PUVA, 9); folliculitis (MD UV-A1, 1; PUVA, 2); sensation of warmth or burning (MD UV-A1, 7); photo-onycholysis (PUVA, 2). | |||
| Comment. PUVA appears to be superior to MD UV-A1 in reducing SCORAD scores and increasing length of remission. In this study, 5-MOP was used instead of 8-MOP. The difference in treatment duration (3 vs 5 weeks) may have influenced the results. Risk of biases: Interventions were allocated by coin toss, but the allocation sequence concealment method was not described (selection bias). Uncertain performance bias in relation to the masking of researchers. Patients were not masked. The second phase of the study was initiated in the event of substantial relapse (SCORAD≥50% of baseline score) or at the request of the patient. | |||
Abbreviations: 5-MOP, 5-methoxypsoralen; 8-MOP, 8-methoxypsoralen; ITT, intention to treat; MD, medium-dose; NB, narrowband; PUVA, psoralen plus UV-A; SCORAD, SCORing Atopic Dermatitis.
Studies Comparing Phototherapy to Other Atopic Dermatitis Treatment Modalities.
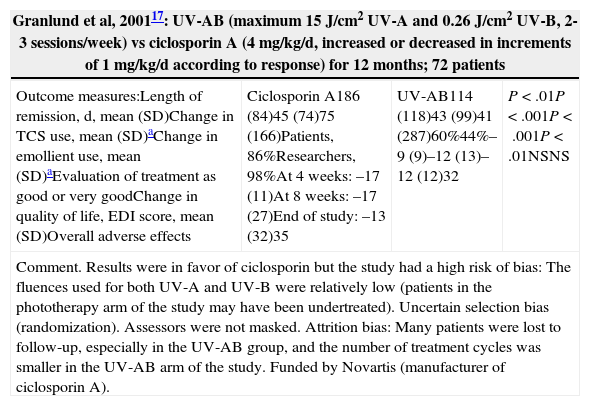
| Granlund et al, 200117: UV-AB (maximum 15J/cm2 UV-A and 0.26J/cm2 UV-B, 2-3 sessions/week) vs ciclosporin A (4mg/kg/d, increased or decreased in increments of 1mg/kg/d according to response) for 12 months; 72 patients | |||
|---|---|---|---|
| Outcome measures:Length of remission, d, mean (SD)Change in TCS use, mean (SD)aChange in emollient use, mean (SD)aEvaluation of treatment as good or very goodChange in quality of life, EDI score, mean (SD)Overall adverse effects | Ciclosporin A186 (84)45 (74)75 (166)Patients, 86%Researchers, 98%At 4 weeks: –17 (11)At 8 weeks: –17 (27)End of study: –13 (32)35 | UV-AB114 (118)43 (99)41 (287)60%44%–9 (9)–12 (13)–12 (12)32 | P<.01P<.001P<.001P<.01NSNS |
| Comment. Results were in favor of ciclosporin but the study had a high risk of bias: The fluences used for both UV-A and UV-B were relatively low (patients in the phototherapy arm of the study may have been undertreated). Uncertain selection bias (randomization). Assessors were not masked. Attrition bias: Many patients were lost to follow-up, especially in the UV-AB group, and the number of treatment cycles was smaller in the UV-AB arm of the study. Funded by Novartis (manufacturer of ciclosporin A). | |||
| Tzung et al, 200614: Group 1: pimecrolimus + NB UV-B vs pimecrolimus. Group 2: pimecrolimus + NB UV-B vs NB UV-B for 6 weeks and 4 weeks of follow-up; 26 patients | ||
|---|---|---|
| Outcome measures:Reduction in EASI scores, % | Group 156 vs 53 (P=.084) | Group 259 vs 55 (P=.059 at week 6 and P=.09 after 4 weeks of follow-up) |
| Reduction in mean pruritus score: NB UV-B + pimecrolimus, 3.1; pimecrolimus, 3; NB UV-B, 3 (P<.001, P=.002, and P=.004, respectively) | ||
| Comment. The pimecrolimus + NB UV-B combination was not demonstrated to be superior. Inclusion and exclusion criteria were not clearly specified. Uncertain risk of selection bias (randomization), performance bias (participants were not blinded), and attrition bias (withdrawals were not explained). Selective reporting: Flares after treatment were measured, although this variable does not appear in the methods section. Measures of dispersion were not provided. | ||
| Valkova and Velkova 200415: UV-AB vs UV-AB + topical corticosteroids (fluticasone or hydrocortisone butyrate), 5 sessions/week for 12 weeks; 31 patients | ||
|---|---|---|
| Reduction in severity score, mean (SD)Reduction in topographic score, mean (SD)Reduction in general score, mean (SD)No. of sessionsUV-B dose, mean (SD), J/cm2Length of remission, mean (SD) | UV-ABFrom 659.8 (62.6) to 132 (28.8) (P<.0001); from 46.2 (4.8) to 9.7 (1.8) (P<.0001); from 360.4 (37.6) to 37.9 (6.7) (P<.0001); 18.3 (0.8) (P=.02);2.3 (0.12) (P=.03);4.5 (0.4) (P=.39) | UV-AB + corticosteroidsFrom 682.5 (50.5) to 136.9 (33.2) (P<.0001);from 43.6 (3.9) to 8.9 (1.8) (P<.0001);from 395.4 (35) to 36.9 (7.3) (P<.0001);15.6 (0.6) (P=.02);1.9 (0.14) (P=.03);4.1 (0.4) (P=.39) |
| Comment. Both treatments induced significant improvement; the addition of topical corticosteroids decreased the total UV-B dose and the duration of treatment without influencing the duration of remissions or the frequency of adverse effects. Neither the amount of corticosteroids applied nor the length of the follow-up period were quantified. High risk of detection bias (masking) and uncertain risk of selection bias (randomization). | ||
Abbreviations: EASI, Eczema Area and Severity Index; EDI, Eczema Disability Index; NB, narrowband; NS, not specified; TCS, topical corticosteroids; UV-AB, combined UV-A and UV-B phototherapy.
Studies Using Less Common Phototherapy Modalities.
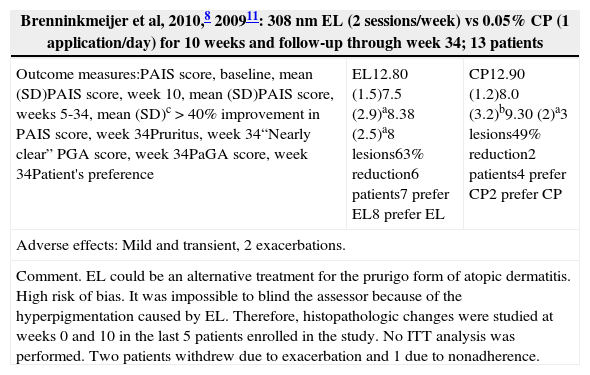
| Brenninkmeijer et al, 2010,8 200911: 308nm EL (2 sessions/week) vs 0.05% CP (1 application/day) for 10 weeks and follow-up through week 34; 13 patients | ||
|---|---|---|
| Outcome measures:PAIS score, baseline, mean (SD)PAIS score, week 10, mean (SD)PAIS score, weeks 5-34, mean (SD)c>40% improvement in PAIS score, week 34Pruritus, week 34“Nearly clear” PGA score, week 34PaGA score, week 34Patient's preference | EL12.80 (1.5)7.5 (2.9)a8.38 (2.5)a8 lesions63% reduction6 patients7 prefer EL8 prefer EL | CP12.90 (1.2)8.0 (3.2)b9.30 (2)a3 lesions49% reduction2 patients4 prefer CP2 prefer CP |
| Adverse effects: Mild and transient, 2 exacerbations. | ||
| Comment. EL could be an alternative treatment for the prurigo form of atopic dermatitis. High risk of bias. It was impossible to blind the assessor because of the hyperpigmentation caused by EL. Therefore, histopathologic changes were studied at weeks 0 and 10 in the last 5 patients enrolled in the study. No ITT analysis was performed. Two patients withdrew due to exacerbation and 1 due to nonadherence. | ||
| Byun et al, 201116: FSL (320-5000nm, 2 sessions/week) + emollient vs emollient for 4 weeks; 38 patients | |||
|---|---|---|---|
| Changes in SCORAD scoresBaselineWeek 4Week 8Subjective assessment of patients, week 4 | FSL + emollient47.8736.81 (–23.1%, P<.01)30.76 (–35.7%, P<.001)Excellent, 6/20; good, 9/20 | Emollient39.4735.3933.85, P=.236Excellent, 7/18; good, 7/18 | |
| Adverse effects in FSL group: erythema, 6/20; dryness, 6/20; pruritus, 4/20; burning sensation, 2/20. Transient exacerbation in 6 patients (first 2 weeks). | |||
| Comment. High risk of performance and detection biases (open study, lack of blinding, unspecified randomization method). Dissimilar baseline characteristics (the baseline SCORAD score difference was 47.87 in the FSL group and 39.79 in the control group). | |||
| Heinlin et al, 20116: sBPT vs NB UV-B, 3-5 sessions/week until end of treatment (35 sessions or early cure), 6months of follow-up; 180 patients | |||
|---|---|---|---|
| Baseline SCORAD, mean (SD)SCORAD session 35, mean (SD)SCORAD 1 mo F/U, mean (SD)SCORAD 6 mo F/U, mean (SD)Baseline SIP, mean (SD)SIP session 35, mean (SD)SIP 1 mo F/U, mean (SD)SIP 6 mo F/U, mean (SD)PGI session 35PGI 1 mo F/UPGI 6 mo F/U | sBPT61.8(14.1)25.6(22)19.0(17.6)18.0(16.4)6.3(8)4.6(6.8)3.7(6.3)4.3(7.4)76.3%73.6%77.5% | NB UV-B61.5(12.4)34.6(22.3)31.1(19.6)25.3(21.9)5.5(5.6)4(5.5)3(3.6)3.3(5.7)55.4%52.6%49% | P=.004P<.0001P=.04P=.77P=.98P=.98P=.99P=.002P=.004P=.002 |
| Comment. sBPT had better results than NB UV-B and remained superior after 6months. Low risk of biases. Assessors were not blinded because they worked at different private practices. | |||
| Selvaag et al, 200519: UV-B vs reflectance-guided UV-B for up to 6 weeks or until a SCORAD score <10; 20 patients | |||
|---|---|---|---|
| UV-B dose, SED (×10mJ/cm2) | UV-BInitial 2.6 (1.9-2.8)Final 9.1 (4.7-14.7)Cumulative 124 (29-186) | Reflectance-guided UV-B3.4 (2.6-5.8)4.9 (3.1-9.2)39 (16-88) | P<.01P<.01 |
| Comment. The radiation dose and the cumulative dose were lower with reflectance-guided UV-B. This was an open study with a high risk of performance and detection biases. No specific data were provided on adverse effects (it was noted that there were no differences). | |||
Abbreviations: CP, clobetasol propionate; EL, excimer laser; FSL, full-spectrum light; F/U, follow-up; ITT, intention to treat; NB, narrowband; sBPT, synchronous balneophototherapy; SED, standard erythema dose; SIP, Sickness Impact Profile; PaGA, Patient's Global Assessment; PAIS, Physician Assessment of Individual Signs; PGA, Physician's Global Assessment; PGI, Patient Global Impression; SCORAD, SCORing Atopic Dermatitis.
aP<.001.
bP<.01.
cP<.05 between EL and CP.
Risk of bias.
+ indicates low risk of bias; ?, unknown risk of bias; –, high risk of bias; 1, generation of random allocation sequence (selection bias); 2, intervention allocation (selection bias); 3, masking of participants and personnel (performance bias); 4, masking of assessors (detection bias); 5, incomplete outcome data (attrition bias); 6, selective reporting (reporting bias); 7, other biases; BJD, British Journal of Dermatology.
Two RCTs24 evaluated the optimal dose of UV-B radiation. The first study compared a UV-B dose of 0.5 times the minimal erythema dose (MED) to a UV-B dose of 1 MED and to visible light. The second study compared a UV-B dose of 0.8 MED to a UV-B dose of 0.4 MED. UV-B radiation was found to be more effective than visible light, but the second study found no differences in efficacy between 0.8 MED and 0.4 MED. In an RCT25 comparing UV-B to UV-AB, statistically significant differences in favor of UV-AB were observed for most variables. In another RCT,26 UV-A was found to be superior to UV-B in the total score and in the overall evaluation, but not in the pruritus score. Two other studies23 compared UV-AB to UV-B and to UV-A, respectively, and found that UV-AB yielded the most favorable results (Table 1).
High-Dose UV-A1 and Medium-Dose UV-A1 (Table 2)An RCT10 carried out in patients with an acute flare of AD found that the decrease in SSS scores at 6 and 15 days after HD UV-A1 phototherapy was statistically significant as compared to UV-AB. Another multicenter RCT18 compared HD UV-A1 (130J/cm2), UV-AB, and 0.5% topical fluocortolone. Significant differences were found in SSS scores on day 5 and day 10 in favor of HD UV-A1 and fluocortolone as compared to UV-AB and in favor of HD UV-A1 as compared to fluocortolone, although no absolute data were reported. A pilot study21 compared MD UV-A1 with HD UV-A1. The decrease in modified SCORAD scores after 3 weeks of treatment was 34.7% in the HD UV-A1 group and 28.2% in the MD UV-A1 group (P>.05), and there were no bilateral differences in time until recurrence or in intensity of recurrence. The cold-light UV-A1 modality dissipates the excessive heat load generated by UV-A1. One study9 found that cold-light UV-A1 was more effective than UV-A1 and UV-AB at clearing lesions and reducing their duration. After 3 weeks, mean SCORAD scores had decreased (P<.05) in all 3 groups—23.3 (10.6) for cold-light UV-A1, 28.8 (6.9) for UV-A1, and 41.4 (9.9) for UV-AB—although the decrease was most striking in the cold-light UV-A1 group (Table 2).
Narrowband UV-B (Table 3)One RCT30 found that NB UV-B was superior to UV-A and to visible light and that the results were maintained at 3 months. In a pilot RCT,27 a half-side comparison study assessed the efficacy of NB UV-B vs MD UV-A1 in patients with AD. In the areas treated with NB UV-B, there was a statistically significant decrease in SSS, Leicester, and lesion severity scores. Another RCT20 included patients who received NB UV-B on one half of the body and UV-A1 on the other. Both treatments resulted in lower scores on the Leicester scale and on a visual analogue scale for pruritus, and there were no significant differences between the groups. In a randomized double-blind controlled crossover trial22 of UV-A1 and NB UV-B, no statistically significant differences were found.
Psoralen Plus UV-A Therapy (Table 4)An RCT28 with low statistical power compared 8-methoxypsoralen (8-MOP) PUVA bath therapy to NB UV-B and did not find any significant differences, although the results were consistent with the possibility of clinically relevant differences. A randomized controlled crossover trial7 compared UV-A1 to oral 5-methoxypsoralen (5-MOP) PUVA therapy. The minimum washout period was 4 weeks and patients were followed up until 12 months after the first treatment. The median length of remission was 4 weeks after UV-A1 and 12 weeks after PUVA. The mean reduction in SCORAD scores was greater after PUVA.
Studies Comparing Phototherapy to Other Treatments for Atopic Dermatitis (Table 5)A multicenter study17 compared ciclosporin A to UV-AB. The mean number of days in remission was 186 (84) after ciclosporin A compared with 114 (118) after UV-AB. Both the patients and the researchers rated ciclosporin A treatment more highly than UV-AB phototherapy. An RCT14 compared 1% pimecrolimus cream to NB UV-B in patients between the ages of 5 and 17 years. Both interventions were beneficial, and concomitant use of both treatments was not found to be superior. Another RCT15 compared UV-AB to UV-AB plus topical fluticasone or topical hydrocortisone butyrate. Significant improvement was seen in both groups. In patients who received a corticosteroid, fewer phototherapy sessions were required and the total mean UV-B dose was lower.
Other Studies (Table 6)One pilot study8 compared 308nm EL with 0.05% clobetasol propionate ointment in patients with the prurigo form of AD. EL yielded optimal results as assessed using the Physician Assessment of Individual Signs (PAIS), the Physician's Global Assessment (PGA), the Patient's Global Assessment (PaGA), and a pruritus scale. In another study,16 FSL phototherapy (wavelengths of 320-5000nm) used in conjunction with an emollient yielded a significantly greater improvement in SCORAD scores at 4 weeks as compared to the emollient alone. One RCT6 compared NB UV-B treatment and synchronous bathing in 10% Dead Sea salt solution—also known as synchronous balneophototherapy (sBPT)—to monotherapy with NB UV-B. sBPT yielded a greater reduction in SCORAD scores than NB UV-B as monotherapy (Table 6) and remained superior 1 month and 6 months after treatment. One RCT19 evaluated the utility of determining UV-B dose on the basis of skin reflectance (reflectance-guided UV-B). At each session, the UV-B dosage used on one side of the body was established on the basis of skin pigmentation, as measured by reflectance, and a conventional UV-B regimen was used on the other side of the body. The cumulative UV-B dosage was lower in the reflectance-guided regimen, and both treatment options had the same clinical outcome.
Registered Studies Currently Underway (Table 7)Of the in-progress RCTs registered with Current Controlled Trials and the World Health Organization's International Clinical Trials Registry Platform, 2 studies met the inclusion criteria. One RCT is comparing NB UV-B as monotherapy to PUVA bath therapy and to NB UV-B plus salt water baths. Another RCT is comparing UV-AB phototherapy to UV-B in patients with various pruritic inflammatory dermatoses, including AD (Table 7).
Studies Currently Underway.
| Identification number | NCT01402414 |
|---|---|
| Title | Narrow-band (NB)-UVB vs Bath-PUVA and NB-UVB Plus Salt Water Baths in Atopic Dermatitis |
| Method | Randomized observer-blinded controlled crossover trial |
| Date registered | July 19, 2011 |
| Current status | Recruiting participants |
| Estimated date of completion | September 2014 |
| Participants | Patients with atopic dermatitis |
| Interventions | Group 1: NB UV-BGroup 2: PUVA bath therapyGroup 3: NB UV-B plus salt water baths |
| Outcome measures | Primary:1. Clinical improvement using SASSAD indexSecondary:2. Evaluation of pruritus and sleeplessness using visual analogue scales (0-10)3. Patient satisfaction, safety and quality of life using Skindex-294. Immunohistochemical and serologic parameters |
| Contact | Sarah Terras, MD (s.terras@klinikum-bochum.de) |
| Identification number | NCT01254240 |
| Title | Efficacy Study of Two Choices of Phototherapy on Itching Skin Diseases |
| Method | Randomized double-blind clinical trial |
| Date registered | December 2, 2010 |
| Current status | Recruiting participants |
| Estimated date of completion | June 2012 (end of data collection on main variable) |
| Participants | Patients with pruritic inflammatory dermatoses (atopic dermatitis, other types of eczema, psoriasis, prurigo simplex subacuta) |
| Interventions | UV-AB vs UV-B phototherapy |
| Outcome measures | Primary: 5-D pruritus score and visual analog scale score at 16 weeks |
| Contact | Guenther Hofbauer, MD, Leading Physician, University Hospital Zurich, Division of Dermatology (hofbauer@usz.ch);Alexander A. Navarini, MD PhD (alexander.navarini@usz.ch) |
Abbreviations: NB, narrowband; PUVA, psoralen plus UV-A; SASSAD, Six Area, Six Sign Atopic Dermatitis; UV-AB, combined UV-A and UV-B phototherapy.
This is the first systematic review on phototherapy in AD to be published in Spanish. We analyzed 21 studies that met the inclusion criteria. These studies included a total of 961 patients, of whom 32 were younger than 18 years of age.
The use of the various phototherapy modalities had a chronological distribution. In the 1980s and 1990s, studies of UV-A, UV-B, and UV-AB phototherapy were predominant. PUVA, NB UV-B, and UV-A1 emerged later, followed by modalities such as EL and FSL.
In some studies, there were statistically significant differences between combined UV-AB phototherapy and UV-A or UV-B phototherapy (Table 1). HD UV-A1 was subsequently found to be superior to UV-AB.10,18 Later studies showed that MD UV-A1 had similar efficacy and fewer side effects than HD UV-A19,21 (Table 2).
The most homogeneous studies are those that compare UV-A1 to NB UV-B (Table 3). Two studies27,30 found NB UV-B to be superior to UV-A1. In an RCT with a large number of participants and low risk of selection bias, the superiority of NB UV-B was maintained after 3 months of follow-up.30 The other 2 studies20,22 that compared UV-A1 to NB UV-B found no important differences between the treatment modalities. It should be noted that an unconventional UV-A1 regimen was used in all 4 studies (2 or 3 weekly sessions instead of 5); the effectiveness of UV-A1 could, therefore, be greater.
A RCT28 with no follow-up period compared 8-MOP PUVA bath therapy to NB UV-B. The results were consistent with clinically relevant differences between the treatments, but the statistical power of the study was low. Another study7 found that oral 5-MOP PUVA therapy yielded a significantly longer remission time than UV-A1, although differences in treatment duration and frequency of application may have influenced the results.
As for the more novel techniques, FSL16 and EL8 have each been evaluated in an RCT and found to have optimal results, although publication bias may have played a role. Similarly, there is scant evidence on combined therapies. One study6 found sBPT to be superior to NB UV-B. In another study,15 the addition of topical corticosteroids to UV-AB therapy was found to reduce UV-B dose and treatment duration. In an RCT14 carried out in children, concomitant use of topical pimecrolimus during NB UV-B therapy did not yield any added benefit. Only 1 RCT17 has compared an immunosuppressive treatment—ciclosporin A—to UV-AB. Ciclosporin A had significantly better results, but the UV-AB doses were suboptimal and the study had a high risk of bias.
All phototherapy modalities were generally described as well-tolerated, although adverse effects were recorded systematically in only a few RCTs. The absence of standardized protocols for the application of these techniques—determination of initial dose according to skin phototype or MED, incremental dose increases, frequency of sessions, etc.—makes it difficult to interpret and compare the results. Given the heterogeneity of the treatments and the reporting deficiencies, we opted not to conduct a meta-analysis.
Quality of EvidenceIn general, the studies had a high or uncertain risk of bias5 and omitted relevant information. In many studies, the number of participants was small, the randomization method was unclear, and patients lost to follow-up were excluded from statistical analysis. The follow-up period was short or nonexistent in most of the studies. In RCTs that used within-patient comparisons, there is a possibility of bias resulting from the possible systemic effect of phototherapy applied to each side of the body.
Patient inclusion and exclusion criteria varied, and the criteria of Hanifin and Rajka were not used in all of the RCTs. In one RCT,14 the inclusion and exclusion criteria were not mentioned explicitly.
Potential Biases in the Review ProcessTo ensure that we compiled as many studies as possible, we reviewed the Global Resource of Eczema Trials database (Centre of Evidence Based Dermatology, http://www.greatdatabase.org.uk/) and found no RCTs that had not been included in our results. However, we restricted our search to studies in English, French, and Spanish. Dr José Manuel Carrascosa, an expert in phototherapy treatment, reviewed our results and detected only the absence of studies in German. Another recent systematic review4 included only 1 article31 in German.
Finally, publication bias is possible, given that most of the studies reported a positive result for the tested treatment modality; however, this bias is less likely because none of the studies were placebo-controlled.
Comparison With Previous Systematic ReviewsThere have been 2 previous systematic reviews on phototherapy in AD. The first review3 did not include studies of PUVA therapy. This review concluded that UV-A1 phototherapy, if available, should be used to treat acute forms of AD and that NB UV-B should be used to treat chronic forms of AD. In our opinion, these conclusions are difficult to justify because these clinical differences are not well established in the cited studies. As for the more recent systematic review,4 there were 4 discrepancies between the RCTs we selected and those selected by the other authors. We included 2 studies8,14 comparing topical therapy to phototherapy that the other review omitted because the authors considered the diagnosis of AD to be doubtful. We also included a study19 that evaluated the utility of reflectance-guided UV-B for reducing UV-B dose. Finally, we omitted 1 study31 included in the other review because it was published in German.
ConclusionsImplications for Practice- •
There is evidence to support the use of NB UV-B and UV-A1 phototherapy in moderate to severe forms of AD. There is scant evidence to support the use of PUVA.
- •
It may be possible to find indications for modalities such as EL in the prurigo form of AD, FSL, and sBPT, but further studies are needed.
- •
Data on the use of phototherapy in childhood AD are limited, and therefore caution must be exercised when this technique is used in children. There is no evidence to support the use of phototherapy in pregnant women with AD.
- •
There are few data on the long-term effects of phototherapy in AD, including possible carcinogenic effects.
- •
We found no RCTs comparing the use of phototherapy to the use of oral corticosteroids.
- •
Only 1 RCT has compared systemic immunosuppressive therapies to phototherapy, and it did not include the modalities for which the strongest evidence is available (NB UV-B and UV-A1).
- •
AD severity assessment criteria, irradiation techniques, and assessment scales and other outcome measures should be standardized; this is the objective of the Harmonizing Outcome Measures for Eczema initiative (http://homeforeczema.org/).
- •
Given the impact of AD, it is surprising that only 2 studies included quality-of-life measures (Skindex and the Eczema Disability Index) and that only 2 other studies included subjective assessment scales.
- •
A minimum follow-up period should be included and tolerability parameters and adverse effects should be recorded.
- •
Results should be reported in accordance with the Consolidated Standards of Reporting Trials statement.
The authors declare that they have no conflicts of interest.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Data confidentialityThe authors declare that no private patient data are disclosed in this article.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
We are grateful to the librarians of our various hospitals for obtaining full-text versions of the articles reviewed.
Please cite this article as: Pérez-Ferriols A, Aranegui B, Pujol-Montcusí JA, Martín-Gorgojo A, Campos-Domínguez M, Feltes RA, et al. Modalidades de fototerapia para el tratamiento de la dermatitis atópica: revisión sistemática de la literatura. Actas Dermosifiliogr. 2015;106:387–401.