Photoallergic contact dermatitis (PACD) to oxybenzone was reported for the first time in 1980. Oxybenzone is the most common photoallergen in the United States and Canada and the fourth most common .in Europe. There are no studies or data on the prevalence of oxybenzone PACD in Argentina.
ObjectiveTo determine the proportion of photosensitive patients with PACD to oxybenzone.
MethodsWe conducted a descriptive cross-sectional study of 35 patients with photosensitivity reactions confirmed by photopatch testing at the Research Center of Hospital Público San Martín in La Plata, Argentina, in 2015 and 2016.
ResultsPACD was identified in 6 patients (17.14%). Five of these (14.28%) had at least one positive reaction to oxybenzone in the photopatch test; 4 had a reaction at irradiated sites only (5 J/cm2 UVA) and one had a reaction at both irradiated and nonirradiated sites.
ConclusionsPACD to sunscreens containing oxybenzone is common and is probably underdiagnosed due to a lack of confirmation by photopatch tests or other diagnostic tools. Sensitization rates vary according to region and are influenced by sunscreen ingredients and variations in the use of sunscreen products, cosmetics, and topical drugs.
La dermatitis por contacto fotoalérgica (DCFA) a oxibenzona fue por primera vez documentada en 1980, siendo hoy el principal fotoalérgeno de Estados Unidos de América, Canadá y el cuarto en Europa. En Argentina no existen datos ni publicaciones con respecto a esta reacción cutánea.
ObjetivoConocer el porcentaje de pacientes con fotosensibilidad afectados con fotoalergia a oxibenzona.
MetodologíaEstudio descriptivo de corte transversal. Un total de 35 pacientes con reacciones fotosensibles con prueba del fotoparche en el Centro de investigación del Hospital Público San Martín, en la ciudad de La Plata, fueron estudiados durante los años 2015 y 2016.
ResultadosSe observó el 17,14% de DCFA, presentando 5 (14,28%) pacientes al menos una reacción positiva a oxibenzona en el test de fotoparche, 4 pacientes solo en la zona irradiada con 5J/cm2 (de UVA) y solo un paciente tanto en la zona irradiada como en la no irradiada.
ConclusionesLa DCFA a protectores solares compuestos por oxibenzona es frecuente y se presume infradiagnosticada debido a la falta de estudios confirmatorios como la prueba del fotoparche. El porcentaje de sensibilización varía de acuerdo con cada región, sobre todo por las distintas composiciones y costumbres de uso en protectores solares, cosméticos y tratamiento tópico.
The province of Buenos Aires, Argentina, has a population of approximately 17 million inhabitants. The Hospital San Martín, located in its capital, La Plata, provides free health care to patients from the entire region. Between latitudes 25 and 35°S of the American continent, a UV index between 12 and 20 has been reported.1 This observation is of relevance given that the intensity of sunlight plays an important role in photosensitivity reactions. This high exposure could explain the large number of cases of photosensitivity, as these levels of sunlight, measured in situ, are sufficient to trigger these processes.2 In this context, those individuals who work outdoors are potential candidates for photoallergic reactions. To date, no research has been published on photoallergic contact dermatitis (PACD) in the region, and so it is of interest to contribute to the overall body of knowledge in the field of photodermatology.
PACD or photoallergy is an immune-mediated reaction to substances in contact with the skin after exposure to sunlight. Chromophores absorb UVA radiation and are transformed into photoallergens.3 After 12-48h, patients in contact with the implicated agent experience an allergic episode in the areas exposed to sunlight.
The main clinical manifestation is acute or chronic eczema on areas of the skin exposed to sunlight and brought into contact with the substance. Moreover, other types of lesion may appear such as lichenoid or urticarial lesions, similar to erythema multiforme or burns.4 To reach a correct diagnosis, photopatch (PP) testing should be performed.5 Photoallergy requires 2 diagnostic techniques, the patch (used for contact dermatitis) and the phototest (used in photodermatology). Few centers in the world use the PP test, and so PACD is assumed to be uncommon.6
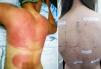
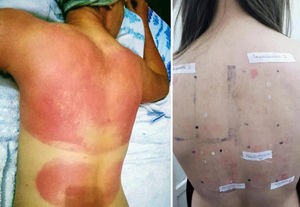
PACD does not develop on areas anatomically protected from the sun (Fig. 1) such as the submaxillary area, the upper eyelids, and the retroauricular crease.7 In Argentina, no epidemiological data are available on the prevalence of PACD and its main photoallergen. Sunscreens are essential for the prevention of photoaging and skin cancer. However, photoallergy as an adverse reaction is usually underdiagnosed,8 and one of the main substances implicated in this condition is, at present, oxybenzone.9
Material and MethodsPopulationA transversal observational study was conducted (protocol number 2919/1189/15 CCIS: Ministry of Health of the Buenos Aires Province) in 445 patients attended in the Skin Allergies Unit of the Dermatology Department of the Hospital San Martín de La Plata, Argentina. Of these patients, a sample of 35 of both sexes aged between 15 and 75 years who had experienced at least 1 episode of photosensitive reaction was selected according to the following inclusion criteria: 1) photosensitive skin rashes; 2) intolerance of sunscreens, fragrances, cosmetics, and local anesthetics; 3) contact eczemas in photoexposed areas; and 4) diagnosis of idiopathic photodermatosis, chronic actinic dermatitis, actinic prurigo, polymorphous light eruption, etc. Patients were excluded for the following reasons: 1) photosensitivity directly linked to systemic diseases (lupus, porphyria) and 2) use of immunosuppressive mediation or topical steroids on the back in the previous month. The following variables were assessed: 1) Sensitizing photoallergens in each patient and 2) other secondary variables such as age and sex.
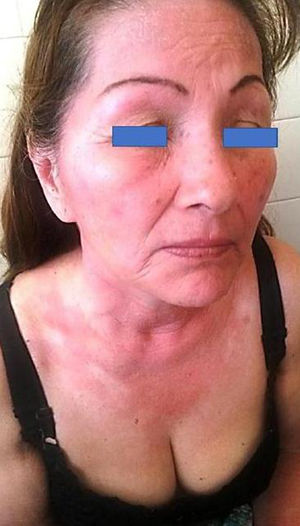
Irradiation DeviceThe irradiation device built to apply the UV dose (in J/cm2) consisted of a hermetic structure that encased a set of 3 pairs of lamps. The UVA lamps manufactured by Philips® covered 3 emission regions whose peak wavelengths (λ) were 350, 365, and 370nm. The source emitted radiation through an aperture that was placed on the area covered by the photopatches. The emission spectrum of each lamp was measured with 2 spectroradiometers: Avantes AvaSpec3648 and Optronic OL-756-2 (Fig. 2). The emission spectrum was integrated from 320 to 400nm to obtain an instantaneous mean (SD) irradiance value of 4.7(0.2)mW/cm2. The corresponding dose was 0.28(0.1)J/cm2 over a 1-minute period. Thus, the time required to deliver a cumulative dose of 5J/cm2 was 18min.
Emission spectrum of the irradiation device with the 6 lamps in operation. Measure of spectral irradiance without the glass protector (black curve) and with the protector in place (blue curve). Exposure time of 18min (for a 5J/cm2 dose) was derived for the measurement made with the glass cover, which filters the UVB component (λ≤320nm) of the source.
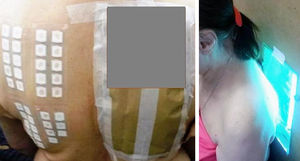
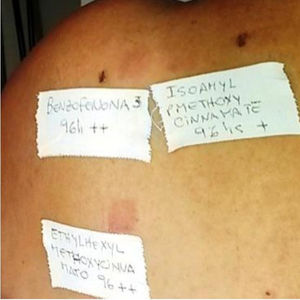
The PP technique used Chemotechnique Diagnostics European Photopatch Extended Series, which comprises 34 photoallergens (of which 19 are compounds included in sunscreens), and IQ-Ultimate patches (Table 1). The procedure used an area of the body not usually exposed to sunlight. Two sets of skin patches were applied, 1 on the right and 1 on the left of the back, with photoallergens diluted in petroleum jelly at the concentration stipulated by the European Committee for Standardization for PP testing. At 48h after placement, 1 of the patches was irradiated (Fig. 3) with the purpose-built device for 18min until the preestablished cumulative dose of 5J/cm2 was reached.8 Readings were made at 30min after irradiation and at 96hours after patch application. Finally a late reading was also taken after 1 week. The findings were assessed using the grading proposed by the International Working Group for Contact Dermatitis Research (mild:+; moderate:++; intense:+++; doubtful:+/–; irritative: IR).
Chemotechnique® Extended European Photopatch Battery and Their Percentage Concentration in Petroleum Jelly.
| Identification No. | Photoallergen Name | Petroleum Jelly Concentration, % |
|---|---|---|
| 1 | Benzophenone-3 | 10.0 |
| 2 | Benzophenone-4 | 2.0 |
| 3 | Enzacamene | 10.0 |
| 4 | Ethylhexyl methoxycinnamate | 10.0 |
| 5 | Octocrylene | 10.0 |
| 6 | Izoamyl p-methoxycinnamate | 10.0 |
| 7 | PABA | 10.0 |
| 8 | Avobenzone | 10.0 |
| 9 | Tinosorb S | 10.0 |
| 10 | Mexoryl XL | 10.0 |
| 11 | Ketoprofen | 1.0 |
| 12 | 2-(4-Diethylamino-2-hydroxybenzoyl)-benzoic acid hexylester | 10.0 |
| 13 | Uvinul T 150 | 10.0 |
| 14 | Tinosorb M | 10.0 |
| 15 | Etophenamate | 2.0 |
| 16 | Uvasorb HEB | 10.0 |
| 17 | Piroxicam | 1.0 |
| 18 | Benzydamine hydrochloridrate | 2.0 |
| 19 | Promethazine hydrochloridrate | 0.1 |
| 20 | Triclocarban | 1.0 |
| 21 | Benzophenone-10 | 10.0 |
| 22 | Ensulizole | 10.0 |
| 23 | Homosalate | 10.0 |
| 24 | Ethylhexyl salicylate | 10.0 |
| 25 | Polysilicone-15 | 10.0 |
| 26 | Disodium phenyl dibenzimidazole tetrasulfonate | 10.0 |
| 27 | Dexketoprofen | 1.0 |
| 28 | Triclosan | 2.0 |
| 29 | Ibuprofen | 5.0 |
| 30 | Diclofenac sodium salt | 5.0 |
| 31 | Fenofibrate | 10.0 |
| 32 | Chlorpromazine hydrochlorate | 0.1 |
| 33 | Olaquindox | 1.0 |
| 34 | Decyl glucoside | 5.0 |
Of the 35 patients assessed with photosensitive reactions, 11 were men (37%) and 24 women (63%). Patient ages ranged from 20 to 72 years, with 85% over 40 years. Eight patients had positive reactions to the PP test, 6 with PACD (17.14%), of which 4 (11.42%) were to oxybenzone and none to octocrylene. Other photoallergic reactions were observed to promethazine, chlorpromazine, benzophenone-10 (sunscreen), cinnamates (ethylhexyl methoxycinnamate, and isoamyl p-methoxycinnamate [sunscreens]), salicylates (ethylhexyl salicylate [sunscreen]), Mexoryl XL® (sunscreen), triclosan (antiseptic), dexketoprofen (nonsteroidal antiinflammatory drug [NSAID]: 2 patients), and fenofibrates (lipid-lowering agent), as shown in Table 2. In particular, benzophenone-10 showed photoallergic reaction in 2 patients with current known relevance. Of the 8 patients, 4 (14.3%) had allergic contact dermatitis (ACD), 3 to decyl glucoside (surfactant in Tinosorb M®) and 1 to oxybenzone. Two of the patients had both ACD and PACD, both caused by sunscreens.
Characteristics of Patients with Positive Photopatch Test.
| Patient | Sex | Age | Positive Results Only in Irradiated Area | Intensity | Relevance | Positive Results in Both Irradiated and Nonradiated Area | Intensity | Relevance | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 50 | Benzophenone-3 | ++ | C | PACD | |||
| Ethylhexyl methoxycinnamate | ++ | C | |||||||
| Isoamyl p-methoxycinnamate | + | C | |||||||
| Benzophenone-10 | ++ | C | |||||||
| Dexketoprofen | ++ | P | |||||||
| Fenofibrate | ++ | CR | |||||||
| 2 | M | 68 | Benzophenone-3 | ++ | C | Decyl glucoside | ++ | C | PACD, ACD, AR |
| 3 | F | 53 | Decyl glucoside | ++ | C | ACD | |||
| 4 | M | 44 | Decyl glucoside | +++ | C | ACD | |||
| 5 | M | 61 | Benzophenone-3 | ++ | C | PACD, AR | |||
| 6 | F | 33 | Benzophenone-3 | ++ | C | PACD, ACD | |||
| Mexoryl XL | + | C | |||||||
| Benzophenone-10 | ++ | C | |||||||
| Ethylhexyl salicylate | ++ | C | |||||||
| Dexketoprofen | + | U | |||||||
| Triclosan | ++ | U | |||||||
| 7 | F | 47 | Benzophenone-3 | ++ | C | PACD | |||
| Benzophenone-10 | ++ | C | |||||||
| 8 | M | 40 | Promethazine hydrochloridrate | ++ | C | PACD | |||
| Chlorpromazine hydrochlorate | ++ | C |
Positive results only in irradiated area and positive results in both irradiated and nonradiated area in indicated patients by number identification number in Table 1.
Relevance: C, current; CR, cross-reaction; P, past; U, unknown.
Diagnoses: ACD, allergic contact dermatitis; AR, actinic reticuloid; PACD, photoallergic contact dermatitis
At least 1 known relevant reaction was identified in these 8 patients, in 7 due to allergens contained in sunscreens and in 1 due to psychoactive drugs (promethazine and chlorpromazine). Reactions to dexketoprofen and triclosan were of unknown relevance or past relevance. On the other hand, fenofibrate was detected as a cross-reaction to oxybenzone in a patient with photoallergy to sunscreen.
Oxybenzone was positive in 5 patients (14.28%), 4 of them with PACD (11.42%). Only 1 female patient had ACD to benzophenone-3, but she also had photoallergy to Mexoryl XL®, ethylhexyl salicylate, and benzophenone-10. In these 5 patients, benzophenone-3 was present in the implicated sunscreens, that is, known relevance was detected.
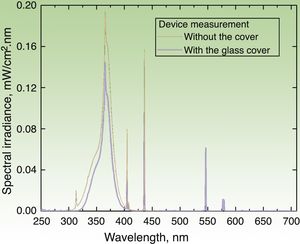
DiscussionSunscreens may become agents responsible for PACD (see for example the patient affected in Figure 4). Currently, sunscreens associated with PACD are usually organic. In the 1980s, a high incidence of PACD to p-aminobenzoic acid (PABA) was reported10; this agent was also related to an increased risk of skin cancer and so is currently not used. In recent years, there have been reports of use of certain PABA esters such as padimate O in sunscreens.11
Patient with photosensitive reaction after exposure to sunlight with pediatric sunscreen. Patient with a history of intolerance to cosmetics. After the test, the patient had a photosensitivity reaction to benzophenone-10 and reaction in the irradiated area and nonirradiated area to benzophenone-3, both of which are components of the sunscreen.
The results of several epidemiological studies in the United States and Europe on PACD differ in prevalence and frequency of the main photoallergens. These differences are likely due to certain factors such as ethnicity, climate, geographical location, habits, duration of exposure to sunlight, and different contact products used, including sunscreens. In the Mayo Clinic in the United States, in a sample of 182 patients, 54 (29.7%) had PACD. The main photoallergens were medications, sunscreens, fragrances, and antisceptics.12
The European Taskforce for Photopatch Testing recommends, by consensus, to perform a PP test with a standard series of photoallergens that contain mainly sunscreens and medications. The results obtained in a multicenter study in 30 dermatology centers between 2008 and 2011 showed that 19.4% of patients had 1 or more reactions. The main causative agents were benzophenone-3 (oxybenzone), octocrylene, and butyl methoxy dibenzoyl methane (avobenzone); the most frequently reported NSAIDs were ketoprofen and ethophenamate. Moreover, unlike in other regions where oxybenzone is the primary causative agent of PACD, in Europe, the main sunscreen implicated was octocrylene, bearing in mind that the substance is similar in chemical structure to ketoprofen, the most widely reported photosensitizer in Europe.13
A recent study of 2577 patients with octocrylene 10% in petroleum jelly, performed in Germany, Austria, and Switzerland, reported only 2 cases (0.08% [95% CI; 0.01%-0.28%]) of relevant positive reactions to this UVB filter in the patch test without irradiation.14 The authors believed that in countries such as Belgium, France, Spain, and Italy, the rates of sensitization to octocrylene are higher because of the more frequent use of topical ketoprofen, which may act as a sensitizer and in turn generate cross reactions. Thus, an increase of 0.7% is observed in ACD and 4% in PACD to octocrylene in the patients studied, but this agent is not considered a main sensitizer.
A study of photopatches in Colombia reported a PACD prevalence of 20% among patients with photosensitivity, a value close to European studies. The main photoallergen was oxybenzone, reported in 15% of patients and 55% of positive cases.15 Based on a previous analysis, a decrease in general sensitization to oxybenzone over 10 years was observed, from 31.7% (2001-2003) to 15% (2011-2013), apparently because this substance was eliminated from many sunscreens used in the region.16
Promethazine and chlorpromazine are antipsychotics that are often implicated in systemic photoallergic reactions when administered orally and also give rise to cross-reactions.17 Likewise, in our study, a case was detected in a patient who only received promethazine. The PP test can not only detect photoallergic reactions but can also be used to diagnose contact dermatitis or dermatitis due to delayed reactions, which appear in both the irradiated and nonirradiated area. For example, sensitization of the nonirradiated area to decyl glucoside, a surfactant used in Tinosorb M®, a broad-spectrum sunscreen with high absorption in the UV range, has been reported.18
Oxybenzone is a compound that absorbs in the range 290-340nm and is widely used in cosmetics, as well as in industrial products (Fig. 5). This aromatic ketone is soluble in organic solvents and insoluble in water. It can cross the skin barrier and its metabolites are excreted in urine after extensive topical application.19 It was named allergen of the year in 2014 by the American Contact Dermatitis Society, and was present in 68% of the 201 sunscreens studied in the United States. Although cross-reactions may occur between oxybenzone and other benzophenones, the mechanism is still not well determined. Benzophenones prevent alteration of the product by UV radiation and are usually used in sunscreens, shampoos, shower gels, hair dyes, soaps, nail varnish, lipstick, and moisturizers. In addition, they are found in industrial products such as paints, varnishes, rubbers, and plastics to increase durability and reduce photodegeneration.20
Moreover, one study reported the case of a tourist who developed PACD on her anterior thighs after exposure to sun on the beach due to contact with a magazine whose ink contained oxybenzone.21 The most frequent clinical manifestations are usually allergic contact eczema and PACD, when used in sunscreens. Other adverse reactions described are contact urticaria and anaphylaxis.22
With regards skin or digestive absorption, given its lipolytic nature, it has been detected in breast milk. Benzophenone-3 has even been found in human urine 4h after topical application.23 This finding led to the investigation of a possible impact on reproduction and ontogenesis in exposed humans and animals. Studies have shown a dose-dependent estrogenic effect of benzophenone and the possibility has even been considered that the agent may induce proliferation of breast cancer in estrogen-positive receptor patients.24,25 On the other hand, studies of environmental impact have detected high concentrations of oxybenzone in regions far from tourist areas, where the agent can affect both fish and coral reefs.26,27
Our results show PACD was detected in 17.14% (6 of 35 patients studied) with photosensitivity over 2 years (2015-2016). Oxybenzone was the most frequently implicated causative organic UVA filter, observed in 5 of the 6 cases: 4 were photoallergic reactions and 1 was allergic contact dermatitis; all had known relevance in the use of sunscreens. The sixth case was a patient with photosensitivity to promethazine and with cross-reaction to chlorpromazine administered orally. Finally, we observed isolated cases of sensitization to decyl glucoside, surfactant of the Tinosorb M® sunscreen included in the battery of photoallergens; this agent does not usually act as a sensitizing chromophore but as a hapten in contact allergy. We did not observe any cases of photoallergy to ketoprofen or octocrylene in our study but there were 2 cases of photoallergy to dexketoprofen with no known relevance.
Photoeducation contemplates increasing awareness of organic sunscreens. With regards the benefit-risk balance of benzophenone-3, it is recommended to use other UVA filters such as avobenzone, particularly in pregnant and breast-feeding women and children.28 Octocrylene is a safe UVB filter, although it should be avoided in children, particularly atopic patients who are allergic to analgesics such as ketoprofen. The use of sunscreens in infants under 6 years of age is not recommended, and in the case of older children, the use of inorganic filters (zinc oxide or titanium dioxide in nanoparticle nonmicronized formulations) is preferred. To make an accurate diagnosis, it is necessary to take into account the responses to questions, clinical signs and symptoms, and the option of performing skin patch tests. A PP test is recommended when a patient presents with a photosensitive reaction and/or when there is suspicion of intolerance to sunscreens.
Our results on the percentage of patients with photoallergy to oxybenzone are consistent with those reported in Colombia. This opens up questions on the differences with respect to other countries. For example, ketoprofen, the main photoallergen in Europe, causes cross-reaction with octocrylene, and so this substance is implicated more frequently than oxybenzone. The use of topical nonsteroidal analgesics is uncommon in South America. However, octocrylene can be found in most commercially available sunscreens.
We believe that constant study of photosensitivity is necessary, particularly in those patients who show intolerance to sunscreens. This would not only provide up-to-date information on the implication of new commercially available products but also help safeguard the health of our patients.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
The authors Ipiña A. and Piacentini R. D. thank the Argentinian National Council for Scientific and Technological Research (CONICET) for their support. We also thank the reviewers for their valuable suggestions and focus in this study.
Please cite this article as: Russo JP, Ipiña A, Palazzolo JF, Cannavó AB, Piacentini RD, Niklasson B. Dermatitis por contacto fotoalérgica a protectores solares con oxibenzona en La Plata, Argentina. Actas Dermosifiliogr. 2018;109:521–528.