The melanocortin-1 receptor (MC1R) is an important risk factor for melanoma due to its role in the production of melanin in response to sun exposure.
ObjectivesTo analyze the phenotypic and histologic characteristics of cutaneous melanoma in patients carrying mutations in MC1R and assess the influence of sun exposure on the occurrence of melanoma.
Material and methodsA total of 224 patients with a diagnosis of melanoma seen in the Department of Dermatology at Hospital General Universitario Gregorio Marañón in Madrid, Spain between September 2004 and December 2009 were included in the study. The genomic sequence of MC1R was analyzed by polymerase chain reaction.
ResultsAt least one of the following MC1R variants was present in 58% of the patients: V60L, V92M, I155T, R160W, D294H, and R163Q. Carriers of those variants had a history of sunburn (P=.018) and melanomas located on areas with intermittent sun exposure (P=.019), and the majority had a diagnosis of superficial spreading melanoma. These associations were especially significant in patients with the R160W and D294H variants. Carriers of R160W also had melanomas associated with melanocytic nevi (P=.028).
ConclusionsThe results of our study suggest that there may be a relationship between the expression of certain MC1R variants and sun exposure, history of sunburn, and skin type. They also indicate a higher frequency of superficial spreading melanomas and melanomas associated with melanocytic nevi in patients carrying certain mutations in MC1R.
El receptor de la melanocortina-1 (MC1R) es un importante determinante del riesgo de melanoma debido a su función en la producción de melanina en respuesta a la exposición solar.
ObjetivosAnalizar las características fenotípicas e histológicas de los pacientes con melanoma cutáneo portadores de mutaciones del MC1R asociadas a riesgo de melanoma y la influencia de la exposición solar en la aparición del melanoma.
Material y métodosSe incluyeron 224 pacientes diagnosticados de melanoma atendidos en el Servicio de Dermatología del Hospital General Universitario Gregorio Marañón (septiembre de 2004 -diciembre de 2009). Se realizó la secuenciación genómica del ADN del MC1R mediante PCR.
ResultadosEl 58% presentaba al menos una de las siguientes variantes de MC1R (V60L, V92M, I155T, R160W, D294H, R163Q). Estos pacientes presentaban antecedentes de quemaduras solares (p=0,018), melanomas localizados en áreas de exposición solar intermitente (p=0,019), con predominio del tipo histológico de extensión superficial. Estas asociaciones fueron especialmente significativas en los portadores de las variantes R160W y D294H. Los portadores de R160W presentaron además melanomas asociados a nevus melanocíticos (p=0,028).
ConclusiónLos resultados obtenidos sugieren que puede existir una relación entre la expresión de determinadas variantes de MC1R y los hábitos de exposición solar, antecedentes de quemadura y tipo de piel del paciente, así como una mayor frecuencia de melanomas de extensión superficial y melanomas asociados a nevus en portadores de ciertas mutaciones de MC1R.
In recent decades, cutaneous melanoma has become the neoplasm with the fastest growing incidence and mortality rate in developed countries.1 Both genetic and environmental factors are involved in the pathogenesis of cutaneous melanoma. UV radiation, one of the most important environmental factors, is estimated to cause at least 65% of malignant melanomas in the white population.2
Fair-skinned individuals whose skin tans poorly or not at all (Fitzpatrick skin phototypes I and II) are more likely to develop cutaneous melanoma. Furthermore, between 3% and 15% of melanomas are familial (occurring in 2 or more members of the same family), suggesting a hereditary predisposition. However, familial melanoma is genetically heterogeneous.3,4 Mutations in 2 high-penetrance susceptibility genes (CDKN2A and CDK4) have been reported in 30%–40% of cases of familial melanoma.3 Thus, other genetic factors associated with a predisposition to melanoma must exist, especially in view of the complexity of human pigmentation and its response to sun exposure.
The melanocortin-1 receptor gene (MC1R) plays a key role in human pigmentation regulation. This gene is located at chromosome 16q24.3 and encodes a G-protein-coupled receptor with 7 membrane-spanning domains. This protein interacts with proopiomelanocortin and corticotropin, stimulating tyrosinase transduction and promoting the synthesis of photoprotective eumelanin.
MC1R is considered to be a major determinant of the risk of melanoma, both sporadic and familial, due to the importance of its role in the production of melanin in response to sun exposure. It is a highly polymorphic gene, and more than 70 variants have been described in the white-skinned population.5 Several studies have demonstrated the functional consequences of some of these variations in the amino acid sequence that constitutes the original (wild-type) protein.6–10 The substitution of a single amino acid (e.g., valine for leucine at position 60 in the V60L variant) can result in a failure to synthesize eumelanin, thus increasing the carrier's risk of burning on exposure to UV radiation and consequently of developing skin tumors. Certain MC1R variants, such as R151C, R160W, and D294E, have been associated with the red hair color phenotype, characterized by fair skin, red hair, multiple solar lentigines, and difficulty in tanning.
Most studies on the association between MC1R and melanoma have been conducted in white populations in Europe and Australia.11–17 However, the phenotypic characteristics of patients with malignant melanomas in Mediterranean countries differ from those of patients of northern European descent. In an earlier case–control study of Spanish patients living in Madrid, we described several novel MC1R variants and confirmed the association between certain variants (V60L, V92M, I155T, R160W, D294H, and R163Q) and a statistically significant risk of developing melanoma.18
In light of the results obtained in that study, we decided to analyze the characteristics of melanoma patients with MC1R mutations, taking as a basic hypothesis that intermittent sun exposure would be of greater importance in the pathogenesis of their melanomas.
The present study was a continuation of the earlier work, and the main objectives were as follows:
- 1.
To analyze the phenotypic characteristics of patients in our hospital who have cutaneous melanoma and carry MC1R variants.
- 2.
To ascertain whether there is a relationship between type of sun exposure and the development of melanoma in carriers of MC1R mutations.
- 3.
To identify significant associations between specific MC1R variants and the epidemiologic, clinical, and histologic characteristics of the patients studied.
A total of 224 patients with primary cutaneous melanoma who were treated in the melanoma unit of the Dermatology Department of the Hospital General Universitario Gregorio Marañón in Madrid, Spain between September 1, 2004 and December 31, 2009 were included in this voluntary study after written informed consent was obtained. The study was approved by the hospital ethics committee. The inclusion criteria were as follows: white patients of Spanish nationality who were diagnosed with primary melanoma during the study period or who had already been diagnosed and attended for a follow-up visit during the study period. We excluded nonwhite patients, foreign nationals, and patients diagnosed with metastatic melanoma of unknown primary origin.
Genomic DNA for sequencing was isolated from peripheral blood lymphocytes (2 tubes of 10cm3 with ethylenediaminetetraacetic acid). The MC1R coding region (16q24.3) was amplified by polymer chain reaction using 2 primer pairs as previously described.14 Sequence analysis was performed with the ABI Prism system (Applied Biosystems) using the BigDye Terminator Cycle Sequencing kit and the ABI 3700 automated DNA sequencer in accordance with the manufacturer's instructions. All the results obtained were confirmed manually.
Patient information was collected using a standardized questionnaire completed by a dermatologist in the presence of the patient. The following epidemiologic data were recorded: age, sex, date and place of birth, sunburn episodes (reported by the patient as blisters or skin pain for at least 48h after exposure and/or the presence of solar lentigines in areas of intermittent exposure to sunlight on physical examination), and a personal and family history of cutaneous or noncutaneous tumors (a family history of melanoma was defined as at least 1 first- or second-degree relative diagnosed with melanoma). The clinical findings recorded were as follows: Fitzpatrick skin phototype, hair color, eye color, and number of nevi and/or solar lentigines. With respect to the melanoma, the following findings were recorded: site, date of diagnosis, histologic type, Breslow thickness, Clark level, histologic evidence of ulceration, and association with a melanocytic nevus.
To analyze the influence of exposure to sunlight, patients were classified into 3 groups according to the exposure of the primary melanoma site, as follows19:
- 1.
No exposure
- 2.
Intermittent exposure
- 3.
Continuous exposure
This was an observational cross-sectional study, patients were included consecutively during the study period, and the statistical analysis was descriptive. Both the descriptive study and the analysis were performed using version 14 of the SPSS statistical analysis software (SPSS Inc). The Pearson χ2 test was used for the univariate analysis of qualitative variables. Analysis of qualitative and quantitative variables was performed using the t test, applying the homogeneity of variance or Bonferroni test when necessary.
LimitationsAlthough our original work on the MC1R mutations in patients with melanoma was a case-control study,18 the difficulty of obtaining a control group of similar size to the group of melanoma patients prevented us from calculating measures of association, such as relative risk or odds ratios.
Potential confounding factors arising from the analysis of so many variables could be resolved with multivariate analysis. However, sample size and the proportions found prevented us from obtaining any significant results with this analysis.
Another difficulty was the determination of the patients’ skin phototype because the Fitzpatrick classification is a rather subjective method based on physical examination and questions such as the following: Have you ever had sunburn? Does your skin tan always, easily, or never?
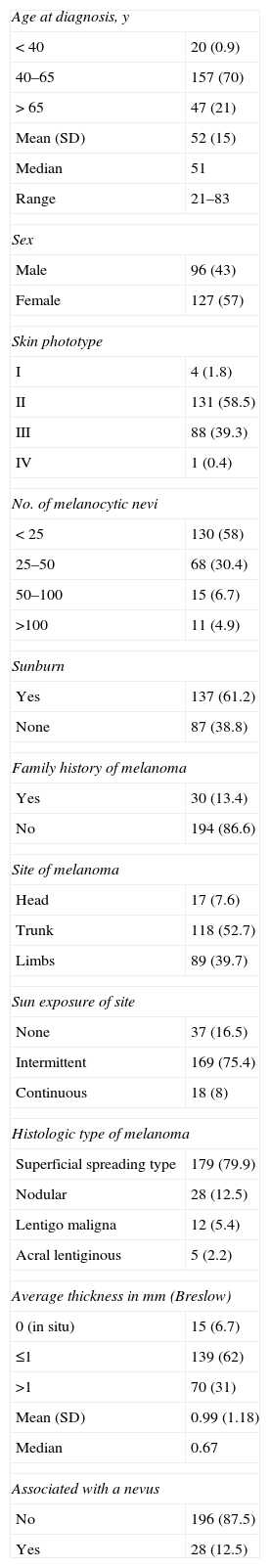
ResultsTable 1 shows the epidemiologic characteristics of the patients included in the study. Seventy percent were middle-aged (between 40 and 65 years of age), 58% had a type II skin phototype, and over 60% had a history of sunburn. Only 11.7% had more than 50 melanocytic nevi. Nine patients (4%) had a history of more than 1 primary melanoma. Thirty patients (13%) reported a family history of melanoma. Participants included 2 parent-and-child pairs and 2 sisters. Interestingly, the MC1R variants were not identical in these intrafamily pairs, and 1 of these patients had no MC1R mutations.
Epidemiologic Characteristics of Patients (n=224) in the Study.a
| Age at diagnosis, y | |
| <40 | 20 (0.9) |
| 40–65 | 157 (70) |
| >65 | 47 (21) |
| Mean (SD) | 52 (15) |
| Median | 51 |
| Range | 21–83 |
| Sex | |
| Male | 96 (43) |
| Female | 127 (57) |
| Skin phototype | |
| I | 4 (1.8) |
| II | 131 (58.5) |
| III | 88 (39.3) |
| IV | 1 (0.4) |
| No. of melanocytic nevi | |
| <25 | 130 (58) |
| 25–50 | 68 (30.4) |
| 50–100 | 15 (6.7) |
| >100 | 11 (4.9) |
| Sunburn | |
| Yes | 137 (61.2) |
| None | 87 (38.8) |
| Family history of melanoma | |
| Yes | 30 (13.4) |
| No | 194 (86.6) |
| Site of melanoma | |
| Head | 17 (7.6) |
| Trunk | 118 (52.7) |
| Limbs | 89 (39.7) |
| Sun exposure of site | |
| None | 37 (16.5) |
| Intermittent | 169 (75.4) |
| Continuous | 18 (8) |
| Histologic type of melanoma | |
| Superficial spreading type | 179 (79.9) |
| Nodular | 28 (12.5) |
| Lentigo maligna | 12 (5.4) |
| Acral lentiginous | 5 (2.2) |
| Average thickness in mm (Breslow) | |
| 0 (in situ) | 15 (6.7) |
| ≤1 | 139 (62) |
| >1 | 70 (31) |
| Mean (SD) | 0.99 (1.18) |
| Median | 0.67 |
| Associated with a nevus | |
| No | 196 (87.5) |
| Yes | 28 (12.5) |
The predominant histologic type was superficial spreading melanoma. In 28 cases (12.5%), the pathology report described a melanocytic nevus associated with the melanoma, although in most cases it did not specify whether these melanocytic nevi were acquired or congenital.
In our series, 75.5% of the tumors were located in areas of intermittent sun exposure.
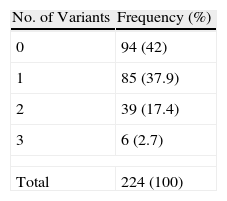
In total, 73% of patients had an MC1R variant, although only 58% had 1 of the 6 variants considered in our previous study to be a risk factor for melanoma.18 Some 20% had 2 or more of these mutations. Table 2 shows the number of MC1R variants associated with a significant risk of melanoma found per patient in our series.
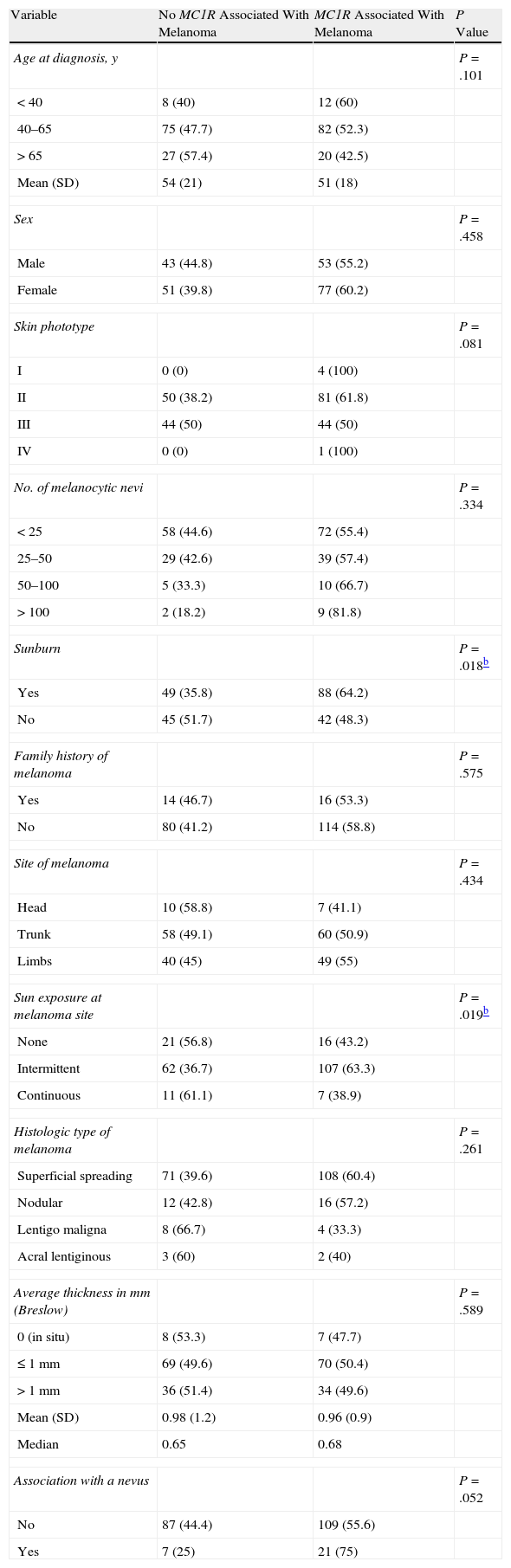
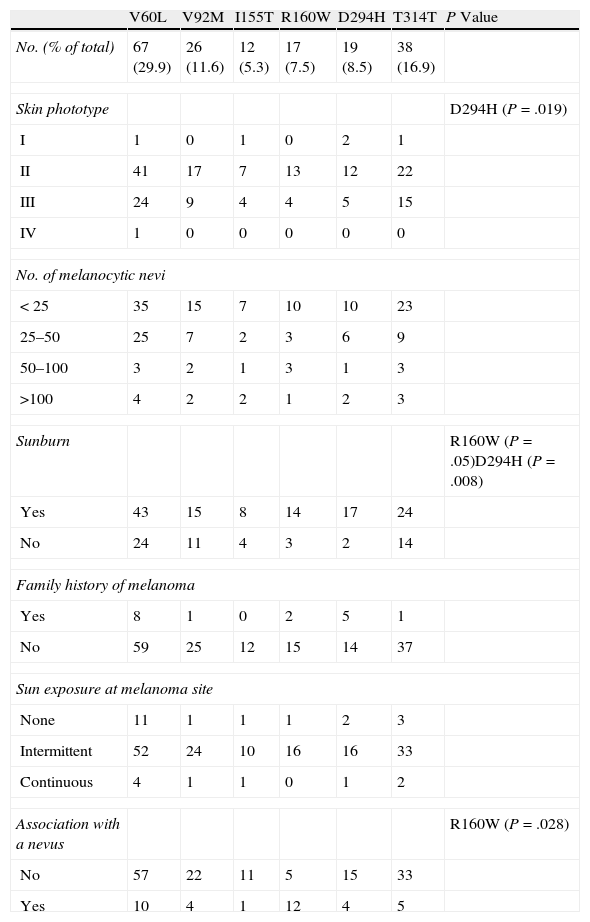
The results of the analysis of the most relevant phenotypic and histologic features and the presence of MC1R variants associated with melanoma are shown in Table 3. These results confirmed the association between the presence of these MC1R variants and fair skin (phototype II), history of sunburn (P=.018), and intermittent sun exposure at the melanoma site (P=.019). These associations were particularly significant in carriers of the R160W and D294H variants (Table 4). Another significant finding was the presence of melanomas associated with a melanocytic nevus in carriers of R160W (P=.028). No significant associations were found between the presence of MC1R mutations and factors such as sex, age, or tumor thickness. Neither were any significant associations found between any of these variables and the number of MC1R variants carried by each patient. However, the tumors of all 6 patients with 3 MC1R variants were located in areas of intermittent exposure to sunlight. Furthermore, 30% of superficial spreading melanomas were found in patients with 2 or more MC1R variants, compared with 6% of nodular melanomas and 8% of lentigo maligna melanomas.
The Association Between Phenotypic and Histologic Features and the Presence of MC1R Variants Associated With Melanoma.a
| Variable | No MC1R Associated With Melanoma | MC1R Associated With Melanoma | P Value |
| Age at diagnosis, y | P=.101 | ||
| <40 | 8 (40) | 12 (60) | |
| 40–65 | 75 (47.7) | 82 (52.3) | |
| >65 | 27 (57.4) | 20 (42.5) | |
| Mean (SD) | 54 (21) | 51 (18) | |
| Sex | P=.458 | ||
| Male | 43 (44.8) | 53 (55.2) | |
| Female | 51 (39.8) | 77 (60.2) | |
| Skin phototype | P=.081 | ||
| I | 0 (0) | 4 (100) | |
| II | 50 (38.2) | 81 (61.8) | |
| III | 44 (50) | 44 (50) | |
| IV | 0 (0) | 1 (100) | |
| No. of melanocytic nevi | P=.334 | ||
| <25 | 58 (44.6) | 72 (55.4) | |
| 25–50 | 29 (42.6) | 39 (57.4) | |
| 50–100 | 5 (33.3) | 10 (66.7) | |
| >100 | 2 (18.2) | 9 (81.8) | |
| Sunburn | P=.018b | ||
| Yes | 49 (35.8) | 88 (64.2) | |
| No | 45 (51.7) | 42 (48.3) | |
| Family history of melanoma | P=.575 | ||
| Yes | 14 (46.7) | 16 (53.3) | |
| No | 80 (41.2) | 114 (58.8) | |
| Site of melanoma | P=.434 | ||
| Head | 10 (58.8) | 7 (41.1) | |
| Trunk | 58 (49.1) | 60 (50.9) | |
| Limbs | 40 (45) | 49 (55) | |
| Sun exposure at melanoma site | P=.019b | ||
| None | 21 (56.8) | 16 (43.2) | |
| Intermittent | 62 (36.7) | 107 (63.3) | |
| Continuous | 11 (61.1) | 7 (38.9) | |
| Histologic type of melanoma | P=.261 | ||
| Superficial spreading | 71 (39.6) | 108 (60.4) | |
| Nodular | 12 (42.8) | 16 (57.2) | |
| Lentigo maligna | 8 (66.7) | 4 (33.3) | |
| Acral lentiginous | 3 (60) | 2 (40) | |
| Average thickness in mm (Breslow) | P=.589 | ||
| 0 (in situ) | 8 (53.3) | 7 (47.7) | |
| ≤1mm | 69 (49.6) | 70 (50.4) | |
| >1mm | 36 (51.4) | 34 (49.6) | |
| Mean (SD) | 0.98 (1.2) | 0.96 (0.9) | |
| Median | 0.65 | 0.68 | |
| Association with a nevus | P=.052 | ||
| No | 87 (44.4) | 109 (55.6) | |
| Yes | 7 (25) | 21 (75) | |
Association Between Phenotypic Features and the Presence of MC1R Variants Associated With Melanoma.
| V60L | V92M | I155T | R160W | D294H | T314T | P Value | |
| No. (% of total) | 67 (29.9) | 26 (11.6) | 12 (5.3) | 17 (7.5) | 19 (8.5) | 38 (16.9) | |
| Skin phototype | D294H (P=.019) | ||||||
| I | 1 | 0 | 1 | 0 | 2 | 1 | |
| II | 41 | 17 | 7 | 13 | 12 | 22 | |
| III | 24 | 9 | 4 | 4 | 5 | 15 | |
| IV | 1 | 0 | 0 | 0 | 0 | 0 | |
| No. of melanocytic nevi | |||||||
| <25 | 35 | 15 | 7 | 10 | 10 | 23 | |
| 25–50 | 25 | 7 | 2 | 3 | 6 | 9 | |
| 50–100 | 3 | 2 | 1 | 3 | 1 | 3 | |
| >100 | 4 | 2 | 2 | 1 | 2 | 3 | |
| Sunburn | R160W (P=.05)D294H (P=.008) | ||||||
| Yes | 43 | 15 | 8 | 14 | 17 | 24 | |
| No | 24 | 11 | 4 | 3 | 2 | 14 | |
| Family history of melanoma | |||||||
| Yes | 8 | 1 | 0 | 2 | 5 | 1 | |
| No | 59 | 25 | 12 | 15 | 14 | 37 | |
| Sun exposure at melanoma site | |||||||
| None | 11 | 1 | 1 | 1 | 2 | 3 | |
| Intermittent | 52 | 24 | 10 | 16 | 16 | 33 | |
| Continuous | 4 | 1 | 1 | 0 | 1 | 2 | |
| Association with a nevus | R160W (P=.028) | ||||||
| No | 57 | 22 | 11 | 5 | 15 | 33 | |
| Yes | 10 | 4 | 1 | 12 | 4 | 5 | |
This is one of the largest studies carried out to date in Spain on the presence of MC1R variants in patients with melanoma. Our findings suggest that there may be an association between the expression of certain MC1R variants and sun exposure, history of sunburn, and skin type. They also indicate a higher frequency of superficial spreading melanomas and melanomas associated with a melanocytic nevus in patients carrying certain MC1R mutations. However, the lack of a control group and the small sample size made it impossible to quantify these associations.
Despite steady progress in the study of the pathogenesis of cutaneous melanoma and the genetic factors involved, these aspects are still poorly understood. Some authors have even questioned the established clinical and histologic classifications of melanoma.20 Alternative classification systems based on the biological behavior of the tumor have been proposed,21,22 and some authors have highlighted the importance of the influence of the patient's immune system and the relationship between the melanoma cells and the surrounding microenvironment in the development of metastases.23 Research aimed at developing future treatments for metastatic melanoma will probably focus on the interaction between biomolecular factors in both the tumor and the patient. What is required in this context is a new melanoma classification that takes into consideration predisposing genetic factors (CDKN2A, MC1R), external factors (exposure to sunlight), and molecular factors (c-Kit, N-Ras, BRAF, and NRas) in addition to the classic histologic factors.3,22
Although most of the studies on genetic predisposition to melanoma have been conducted in white populations in northern Europe and Australia, fortunately, more and more studies are now being carried out in Mediterranean countries.14,15,18,19,24,25 It is not appropriate to extrapolate results obtained in Sweden or Australia to the Spanish population since the epidemiologic profile of these patients differs from that of Spanish patients. A study by Scherer et al.26 examining differences between MC1R mutations isolated in Spanish and German patients detected a greater frequency of mutations not associated with the red hair color phenotype in the Spanish population, and in particular of V60L. In a recent meta-analysis, Kanetsky et al.10 found that certain MC1R mutations were associated with increased risk of melanoma in people with no history of sunburn, those who tan easily, and individuals with dark skin and hair. The results of that study confirm the variable influence of different MC1R mutations in different populations.
The typical profile of a patient with melanoma in Spain is a man or woman aged 40–65 years with chestnut or dark brown hair who does not tan easily, has a history of sunburn and, in our experience, has few melanocytic nevi.27 Histologic type also appears to be influenced by these factors, with a greater number of superficial spreading melanomas among carriers of MC1R mutations. Moreover, MC1R variants were less common in patients with melanomas in sites never exposed or continuously exposed to sunlight (lentigo maligna melanoma), a finding that supports the hypothesis that not all melanomas are the result of intermittent exposure to sunlight.
In conclusion, patients with cutaneous melanoma carrying certain MC1R variants have certain distinctive phenotypic characteristics (fair skin, difficulty in tanning, a history of sunburn, few or no melanocytic nevi, melanoma located in areas of intermittent sun exposure) and histologic characteristics (superficial spreading melanoma, melanoma in association with a nevus). The presence of these variants, together with intermittent exposure to sunlight, is probably key factor in the development of melanomas in Spain. Our results should be confirmed by case–control studies. Given the complexity of cutaneous melanoma, any contributions that can help us better understand its pathogenesis are of significant value to both the scientific community and the population as a whole.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Avilés JA, et al. Características fenotípicas e histológicas de los pacientes con melanoma cutáneo en función de los polimorfismos del MC1R. Actas Dermosifiliogr. 2012;103:44–50.