Pemphigus vulgaris (PV) is an uncommon, serious disease that is treated with systemic corticosteroids and corticosteroid-sparing agents.
ObjectivesTo describe and analyze the demographic and clinical characteristics of patients with PV.
Material and methodsRetrospective cohort study of adults diagnosed with PV over a period of 12 years.
ResultsPV presented with mucosal lesions in 20 of the 32 patients studied (63%); the most common site was the oral mucosa followed by the vulva. Mucosal involvement was more common in women (P=.03). Lesions were found at more than 1 mucosal site in patients whose disease began in the mucosa, independently of age or sex (P=.003). Disease onset before the age of 40 years was associated with generalized skin lesions (P=.003), a need for corticosteroid-sparing therapy (P=.05), and refractory PV (P=.02). Azathioprine was the most widely prescribed corticosteroid-sparing agent (in 22 patients). Eight patients (25%) were dependent on corticosteroids and disease recurred in 26 (81%). Complete remission, with or without treatment, was achieved in 15 patients (47%). Patients remained disease-free for a median of 14 months, and 2 patients died (6%).
ConclusionOnset before the age of 40 years could be a sign of poor prognosis in patients with PV, as it was significantly associated with a higher risk of generalized skin involvement, a need for corticosteroid-sparing therapy, and refractory disease.
El pénfigo vulgar (PV) es una enfermedad infrecuente y grave. Su tratamiento consiste en el uso de glucocorticoides sistémicos (GS) junto a fármacos ahorradores (FA) de los mismos.
ObjetivosDescribir y analizar las características demográficas y clínicas de pacientes con PV.
Materiales y métodosEstudio de cohorte retrospectivo, en adultos con PV, en un periodo de 12 años.
ResultadosSe incluyeron 32 pacientes. En 20 pacientes (63%) el inicio fue en mucosas; la región oral fue la más afectada, seguida de la vulvar. La afectación mucosa fue más frecuente en mujeres (p=0,03). Los pacientes cuyo PV comenzó en esta localización afectaron en su evolución a más de una mucosa, de forma independiente de la edad y el sexo (p=0,003). El comienzo de la enfermedad antes de los 40años se asoció con un compromiso cutáneo generalizado (p=0,003), con la necesidad de tratamiento con FA (p=0,05) y con cierta refractariedad a los tratamientos (p=0,02). La azatioprina fue el FA más prescrito (n=22). Se observó corticodependencia en 8 pacientes (25%) y recaídas en 26 (81%). Se alcanzó una remisión completa con/sin tratamiento en 15 de los casos (47%). La mediana del tiempo libre de enfermedad fue de 14meses y la mortalidad, del 6% (n=2).
ConclusiónEl inicio del PV antes de los 40años podría ser un factor de mal pronóstico, ya que en estos pacientes observamos una mayor probabilidad de presentar compromiso cutáneo generalizado, requerimiento de FA y refractariedad al tratamiento.
Pemphigus vulgaris (PV) is a chronic autoimmune blistering disease that is more common in patients in the fifth and sixth decades of life.1,2
The disease is characterized by flaccid blisters that rupture easily, leaving painful erosions.1 Baum et al.3 observed that PV presents as mucosal lesions in 41.7% of patients, as simultaneous mucosal and cutaneous lesions in 37.4%, and as cutaneous lesions in 20.4%.
Diagnosis is based on examination of a biopsy specimen with hematoxylin-eosin staining and direct immunofluorescence.4
If left untreated, PV can be fatal. Before the introduction of systemic corticosteroids, mortality rates ranged between 60% and 90%.5 Now, thanks to the use of these drugs and corticosteroid-sparing agents, mortality stands at around 10%.6 The starting dose of systemic corticosteroids is empirical and is usually based on clinical experience and disease severity. It typically ranges from 0.5mg/kg/d to 2mg/kg/d.7,8 Corticosteroid-sparing agents, such as methotrexate, azathioprine, mycophenolate mofetil (MM), and rituximab increase the effectiveness of systemic corticosteroids, reduce the need for these drugs, and prevent adverse effects.9,10 Intravenous pulse therapy with methylprednisolone, cyclophosphamide, or gammaglobulin can be used in patients with severe PV.10,11 The use of systemic corticosteroids and corticosteroid-sparing agents has changed over time. The first-line treatment for 15 years consisted of systemic corticosteroids and, where necessary, corticosteroid-sparing agents such as azathioprine or MM.8 The latest recommendations on the diagnosis and management of PV published by an international panel of experts in 2018 establish systemic corticosteroids and rituximab as first-line treatments and azathioprine and MM as first-line corticosteroid-sparing agents.12,13
Patients typically develop PV- and treatment-related complications during the course of their disease.14,15
The aims of this study were to analyze the demographic and clinical characteristics of patients with PV, describe treatments, response to treatment, and clinical course, and analyze factors associated with relapse and refractory disease.
Material and MethodsDesignAnalytical observational study of a retrospective cohort at Hospital Italiano de Buenos Aires, a tertiary hospital in Buenos Aires, Argentina.
PopulationWe studied patients aged over 18 years of age with a diagnosis of PV confirmed by hematoxylin-eosin staining and direct immunofluorescence between September 1, 2005 and August 31, 2017. To be included, the patients had to have a minimum follow-up of 6 months.
Patients with a concomitant blistering disease or who were participating in another research study were excluded.
Exploratory Measures and VariablesDemographic and clinical variables and information on treatment and clinical course were obtained from the patients’ electronic medical records. The principal investigator validated the diagnosis of PV in all cases by reviewing clinical course and test results.
A record was made of age at diagnosis, sex, presenting form of PV, mucosal involvement, cutaneous sites affected, starting dose, dose changes, total treatment duration, systemic corticosteroid discontinuation, corticosteroid dependence, use of corticosteroid-sparing agents, complications, hospital admissions, relapses, complete remission on or off therapy, and overall mortality.
Endpoints were defined as per the Consensus Statement on Definitions of Disease, End points, and Therapeutic Response for Pemphigus16 and the 2018 expert recommendations13 (Table 1). As there is no widely accepted definition of corticosteroid dependence for PV, we used the definition applied to other diseases.17 Intercurrent PV-related conditions and adverse treatment effects were defined as complications. These events were classified as cutaneous or extracutaneous infections, noninfectious skin complications, endocrinological, hematological, bone/skeletal, ophthalmological, psychiatric, digestive, hepatic, constitutional, cardiovascular, renal, or urological complications, lipid alterations, electrolyte disturbances, and other.
Definition of Variables: Consensus Statement on Definitions of Disease, End Points, and Therapeutic Response for Pemphigus and 2018 Expert Panel Recommendations.
| Complete remission on/off therapy13,16 | Absence of lesions for at least 2 months while the patient was receiving equivalent doses of minimal therapy or was off therapy |
| Relapse13,16 | Appearance of ≥ 3 lesions that did not heal spontaneously in 1 week or extension of established lesions |
| Minimal therapy13,16 | Doses ≤ 10mg/d of prednisone (or equivalent) or half the full dose of a corticosteroid-sparing agent for 2 months |
| Corticosteroid dependence17 | Impossibility of reducing corticosteroid dose on minimal therapy without patient experiencing relapse |
| Refractory disease13,16 | Relapse despite full doses for a minimum period of time (variable according to drug) |
The Shapiro–Wilk test was used to check the normality of data distribution. The data were nonnormally distributed and the results are therefore expressed as median and interquartile range (IQR). Categorical variables are described as absolute and relative frequencies. Associations were analyzed using the Fisher exact test for categorical variables and the Mann–Whitney U test for quantitative variables. Statistical significance was set at a P value of less than .05. The statistical analyses were performed in STATA (version 14.0).
The study was approved by the ethics committee at Hospital Italiano de Buenos Aires (protocol No. 2980).
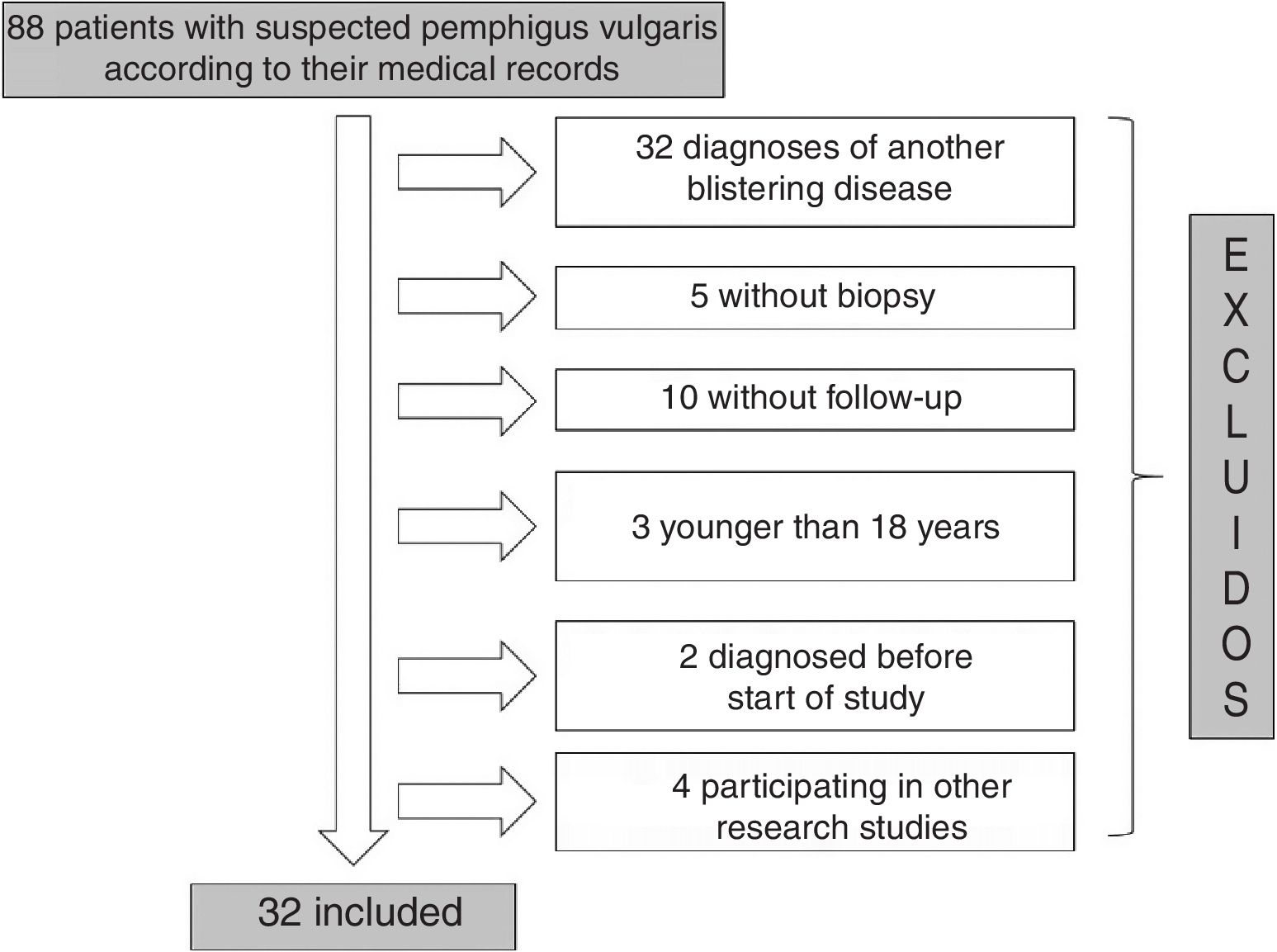
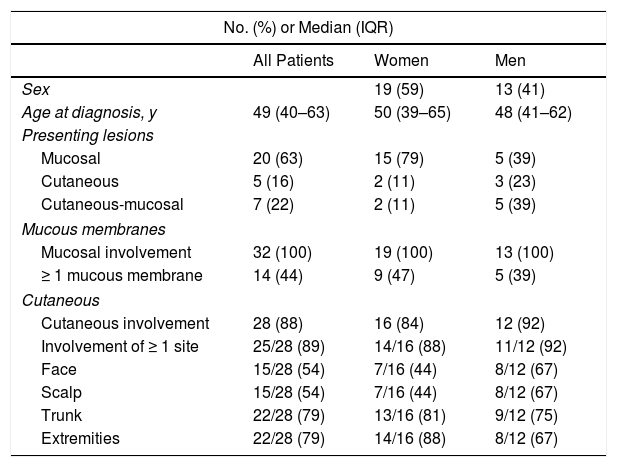
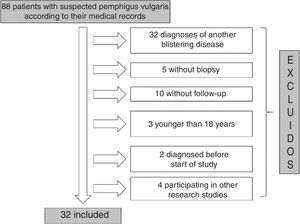
ResultsOf the 88 patients with a suspected diagnosis of PV in their medical records, 32 met the inclusion criteria (Fig. 1). Nineteen (59%) were women and the median age at diagnosis for both men and women was 49 years (IQR, 40–63 years) (Table 2).
Demographic and Clinical Characteristics of Patients With Pemphigus Vulgaris (n=32).
| No. (%) or Median (IQR) | |||
|---|---|---|---|
| All Patients | Women | Men | |
| Sex | 19 (59) | 13 (41) | |
| Age at diagnosis, y | 49 (40–63) | 50 (39–65) | 48 (41–62) |
| Presenting lesions | |||
| Mucosal | 20 (63) | 15 (79) | 5 (39) |
| Cutaneous | 5 (16) | 2 (11) | 3 (23) |
| Cutaneous-mucosal | 7 (22) | 2 (11) | 5 (39) |
| Mucous membranes | |||
| Mucosal involvement | 32 (100) | 19 (100) | 13 (100) |
| ≥ 1 mucous membrane | 14 (44) | 9 (47) | 5 (39) |
| Cutaneous | |||
| Cutaneous involvement | 28 (88) | 16 (84) | 12 (92) |
| Involvement of ≥ 1 site | 25/28 (89) | 14/16 (88) | 11/12 (92) |
| Face | 15/28 (54) | 7/16 (44) | 8/12 (67) |
| Scalp | 15/28 (54) | 7/16 (44) | 8/12 (67) |
| Trunk | 22/28 (79) | 13/16 (81) | 9/12 (75) |
| Extremities | 22/28 (79) | 14/16 (88) | 8/12 (67) |
Abbreviation: IQR, interquartile range.
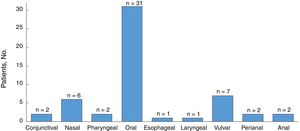
PV presented as mucosal lesions in 20 patients (63%), as mucosal and cutaneous lesions in 7 (22%), and as cutaneous lesions in 5 (16%). Of the 20 patients with mucosal lesions only at onset, 18 had oral lesions and 6 had genital lesions. There was also 1 case each of pharyngeal, esophageal, and nasal involvement. Five patients had lesions at more than 1 mucosal site. Of the 7 patients with concomitant cutaneous and mucosal lesions, 2 had generalized lesions, 4 had scalp lesions, 3 had trunk lesions, 2 had lesions on the upper extremities and pinnae, and 1 had paronychia. Of the 5 patients with cutaneous lesions only at onset, 3 had lesions on the trunk, 2 had lesions on the scalp, and 1 had lesions on the pinnae and upper extremities. Twenty-five patients (78.1%) had simultaneous involvement of more than 1 region.
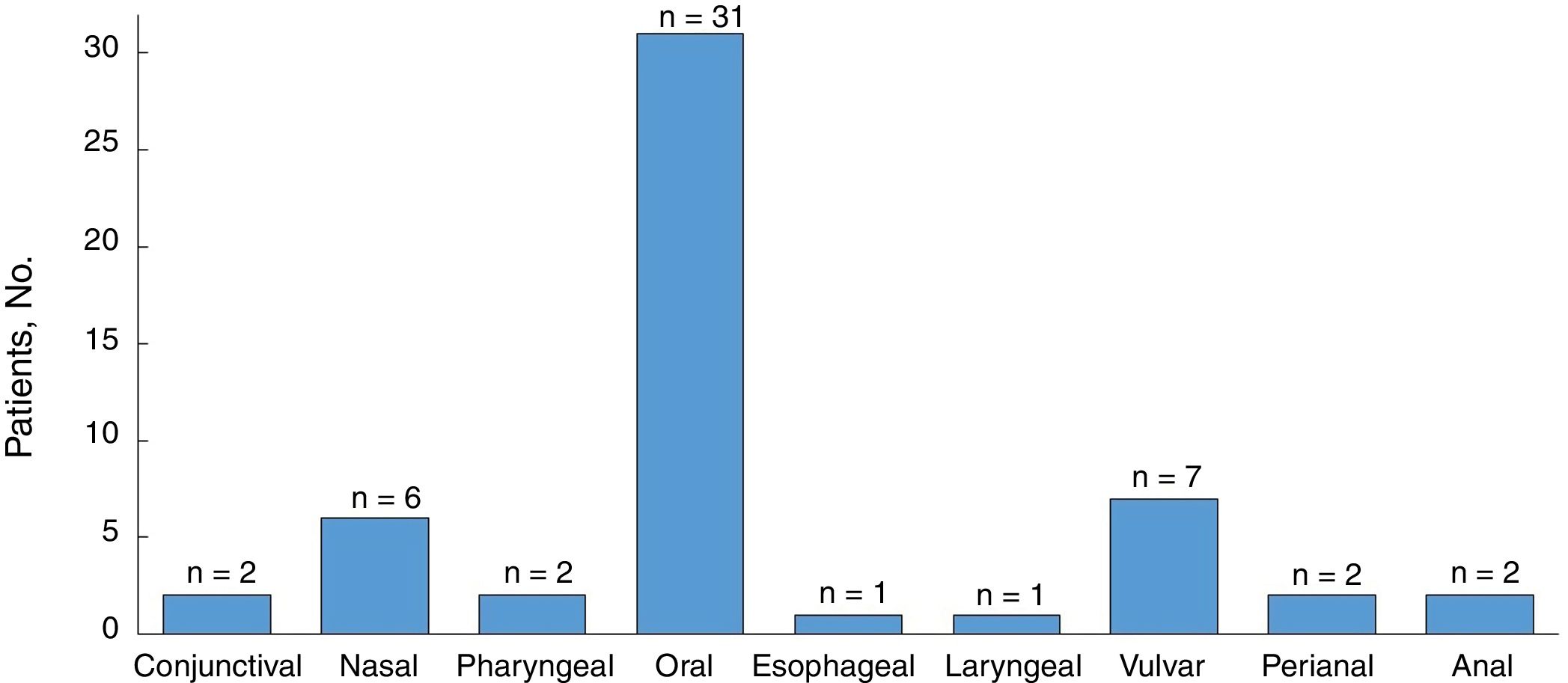
All the patients developed mucosal lesions during the course of disease. Oral lesions were the most common lesions observed (97%) followed by genital lesions (28%) (Fig. 2); 44% of patients had lesions involving more than 1 mucous membrane.
Twenty-eight patients (88%) developed cutaneous lesions during follow-up. The most frequently affected sites were the trunk and the extremities (22/28 patients [79%]).
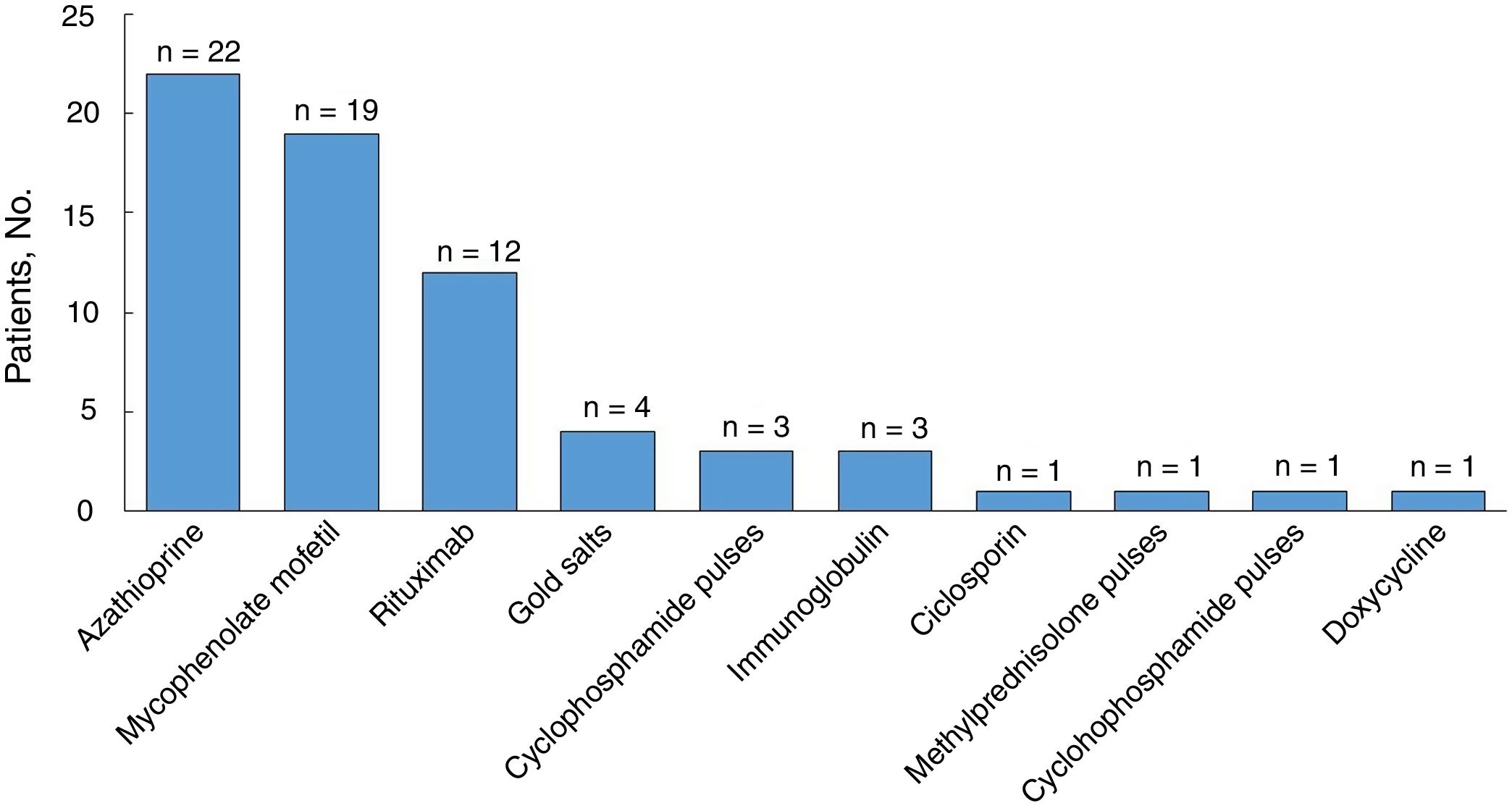
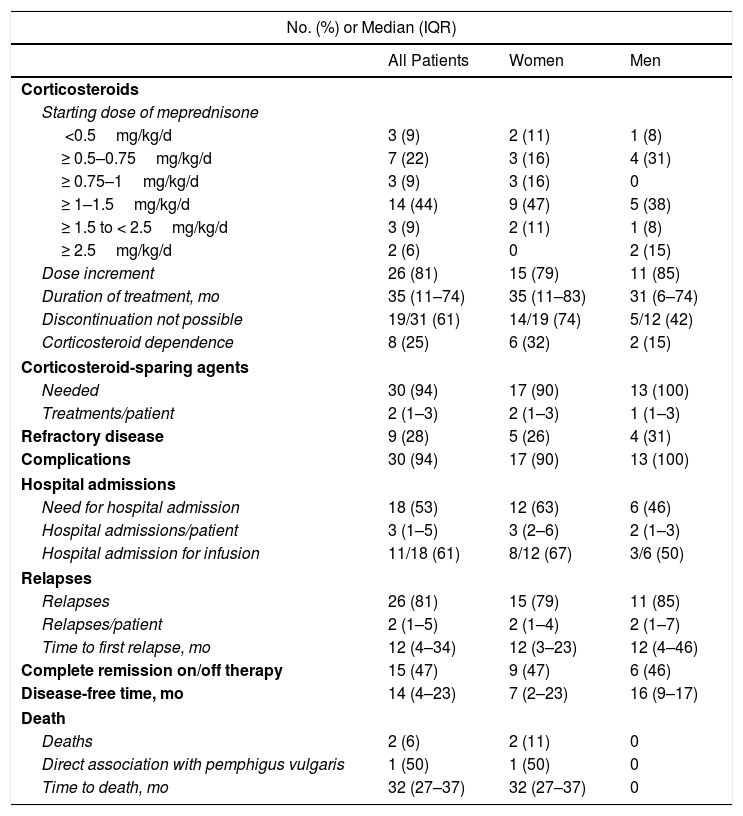
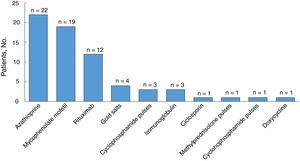
All the patients were started on systemic corticosteroids (Table 3) and the most common starting dose (in 44% of patients) was ≥ 1–1.5mg/kg/d. Twenty-six patients (81%) required a dose increment during follow-up and the median duration of treatment was 35 months (IQR, 11–74 months). Nineteen of 31 patients (61%) were still on systemic corticosteroids at the last visit. Eight of the 32 patients were corticosteroid dependent and 30 (94%) were treated with corticosteroid-sparing therapy (Fig. 3). Azathioprine was the most common first-line corticosteroid-sparing agent (15 patients [50%]), followed by MM (12 patients [40%]). Rituximab was the main second-line treatment, followed by MM and azathioprine. Overall, azathioprine (22/30 patients [73%]), followed by MM (19/30 [63%]), was the most commonly used corticosteroid-sparing agent.
Treatment and Disease Course in Patients With Pemphigus Vulgaris (n=32).
| No. (%) or Median (IQR) | |||
|---|---|---|---|
| All Patients | Women | Men | |
| Corticosteroids | |||
| Starting dose of meprednisone | |||
| <0.5mg/kg/d | 3 (9) | 2 (11) | 1 (8) |
| ≥ 0.5–0.75mg/kg/d | 7 (22) | 3 (16) | 4 (31) |
| ≥ 0.75–1mg/kg/d | 3 (9) | 3 (16) | 0 |
| ≥ 1–1.5mg/kg/d | 14 (44) | 9 (47) | 5 (38) |
| ≥ 1.5 to < 2.5mg/kg/d | 3 (9) | 2 (11) | 1 (8) |
| ≥ 2.5mg/kg/d | 2 (6) | 0 | 2 (15) |
| Dose increment | 26 (81) | 15 (79) | 11 (85) |
| Duration of treatment, mo | 35 (11–74) | 35 (11–83) | 31 (6–74) |
| Discontinuation not possible | 19/31 (61) | 14/19 (74) | 5/12 (42) |
| Corticosteroid dependence | 8 (25) | 6 (32) | 2 (15) |
| Corticosteroid-sparing agents | |||
| Needed | 30 (94) | 17 (90) | 13 (100) |
| Treatments/patient | 2 (1–3) | 2 (1–3) | 1 (1–3) |
| Refractory disease | 9 (28) | 5 (26) | 4 (31) |
| Complications | 30 (94) | 17 (90) | 13 (100) |
| Hospital admissions | |||
| Need for hospital admission | 18 (53) | 12 (63) | 6 (46) |
| Hospital admissions/patient | 3 (1–5) | 3 (2–6) | 2 (1–3) |
| Hospital admission for infusion | 11/18 (61) | 8/12 (67) | 3/6 (50) |
| Relapses | |||
| Relapses | 26 (81) | 15 (79) | 11 (85) |
| Relapses/patient | 2 (1–5) | 2 (1–4) | 2 (1–7) |
| Time to first relapse, mo | 12 (4–34) | 12 (3–23) | 12 (4–46) |
| Complete remission on/off therapy | 15 (47) | 9 (47) | 6 (46) |
| Disease-free time, mo | 14 (4–23) | 7 (2–23) | 16 (9–17) |
| Death | |||
| Deaths | 2 (6) | 2 (11) | 0 |
| Direct association with pemphigus vulgaris | 1 (50) | 1 (50) | 0 |
| Time to death, mo | 32 (27–37) | 32 (27–37) | 0 |
Abbreviation:IQR, interquartile range.
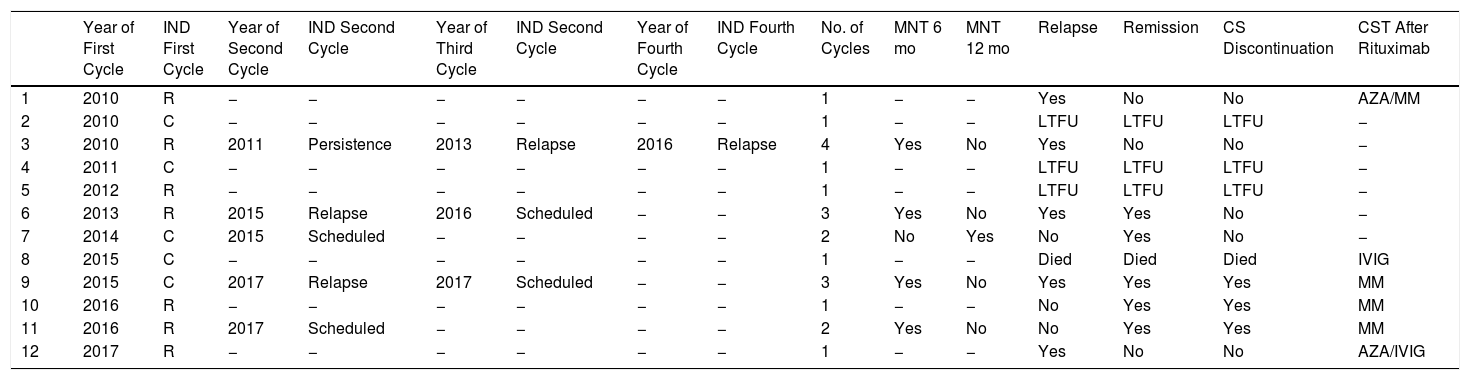
Rituximab was used in 40% of the patients (12/30) who required corticosteroid-sparing therapy. Seven of the patients (58%) had refractory disease and 5 (42%) were corticosteroid dependent. Rituximab was prescribed as first-line therapy in 1 patient and as second-line in 5. In the rest of the patients, it was used after several other agents had been attempted. Seven patients were treated with rituximab for the first time before 2015 at a dosage of 375mg/m2 per week for 4 weeks. Five patients were prescribed rituximab for the first time after 2015 and were treated with a dose of 1g on days 0 and 15. Seven of the 12 patients received just 1 cycle of rituximab. Four patients were treated with 2 or 3 cycles (2 cases each) and 1 with 4 cycles. A single maintenance dose of rituximab was administered to 4 patients at 6 months: 1 had persistent erosions on the oral mucosa and the other 3, none of whom showed any signs of relapse, received the dose as part of the treatment schedule. One patient received a single maintenance dose at 12 months, also as part of the treatment schedule. Six patients treated with rituximab required subsequent treatment with other corticosteroid-sparing agents and the most common drug was MM. Systemic corticosteroid discontinuation was only possible in 3 of the patients treated with rituximab (Table 4).
Treatment With Rituximab (n=12).
| Year of First Cycle | IND First Cycle | Year of Second Cycle | IND Second Cycle | Year of Third Cycle | IND Second Cycle | Year of Fourth Cycle | IND Fourth Cycle | No. of Cycles | MNT 6 mo | MNT 12 mo | Relapse | Remission | CS Discontinuation | CST After Rituximab | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2010 | R | − | − | − | − | − | − | 1 | − | − | Yes | No | No | AZA/MM |
| 2 | 2010 | C | − | − | − | − | − | − | 1 | − | − | LTFU | LTFU | LTFU | − |
| 3 | 2010 | R | 2011 | Persistence | 2013 | Relapse | 2016 | Relapse | 4 | Yes | No | Yes | No | No | − |
| 4 | 2011 | C | − | − | − | − | − | − | 1 | − | − | LTFU | LTFU | LTFU | − |
| 5 | 2012 | R | − | − | − | − | − | − | 1 | − | − | LTFU | LTFU | LTFU | − |
| 6 | 2013 | R | 2015 | Relapse | 2016 | Scheduled | − | − | 3 | Yes | No | Yes | Yes | No | − |
| 7 | 2014 | C | 2015 | Scheduled | − | − | − | − | 2 | No | Yes | No | Yes | No | − |
| 8 | 2015 | C | − | − | − | − | − | − | 1 | − | − | Died | Died | Died | IVIG |
| 9 | 2015 | C | 2017 | Relapse | 2017 | Scheduled | − | − | 3 | Yes | No | Yes | Yes | Yes | MM |
| 10 | 2016 | R | − | − | − | − | − | − | 1 | − | − | No | Yes | Yes | MM |
| 11 | 2016 | R | 2017 | Scheduled | − | − | − | − | 2 | Yes | No | No | Yes | Yes | MM |
| 12 | 2017 | R | − | − | − | − | − | − | 1 | − | − | Yes | No | No | AZA/IVIG |
Abbreviation: AZA, azathioprine; C, corticosteroid dependence; CST, corticosteroid-sparing therapy; IND, indication; IVIG, intravenous immunoglobulin; LTFU, loss to follow-up; MM, mycophenolate mofetil; MNT, maintenance; R, refractory disease; SC, systemic corticosteroid.
Nine (28%) of the 32 patients had refractory PV and 26 (81%) experienced relapse. The median time from diagnosis to the first relapse was 12 months (IQR, 6–34 months). Five (42%) of the 12 patients treated with rituximab experienced relapse: 4 after the first cycle and 1 after the second.
Complete remission on or off therapy was achieved by 47% of patients (n=15), with a median disease-free time of 14 months (IQR, 4–23 months) (Table 3). Of the patients who achieved remission without rituximab (10/15 [60%]), 60% (6/10) had been treated with azathioprine, 20% (2/10) with MM, and 10% (1/10) with azathioprine and MM. One patient (10%) did not require corticosteroid-sparing therapy. Five patients achieved remission with rituximab. Of these, 4 had been previously treated with azathioprine and MM. The median time from the first infusion to disease remission was 4 months (IQR, 3–8 months).
During follow-up, 53% of patients (n=18) needed to be admitted to hospital at least once. There were 44 admissions in total (median of 3 per patient, [IQR, 1–5 admissions]). Nineteen were 1-day admissions for infusion and the other 25 were admissions due to PV or complications. The median duration in these cases was 8 days (IQR, 2–15 days).
A total of 190 complications were reported for 30 of the 32 patients (Table 5). Endocrinological complications were the most common (29/190 [15%]), followed by bone/skeletal complications (27/190 [14%]), which consisted of 15 cases of osteopenia/osteoporosis and 5 cases of vertebral fracture. These complications occurred despite the use of preventive measures, namely early evaluation by an endocrinologist and prescription of vitamin D (23/32 [72%]), calcium (17/32 [53%]), or bisphosphonates (17/32 [53%]), as appropriate. Extracutaneous infections were the third most common complication. Two patients died during follow-up: one of end-stage chronic kidney failure and the other of community-acquired pneumonia.
Complications: 190 Events in 30/32 Patients.
| Type | No. |
|---|---|
| Cutaneous infections | 17 |
| Cellulitis, tinea | 3 |
| Impetiginization of pemphigus erosions, herpes zoster, pityriasis versicolor, onychomycosisa | 2 |
| Herpes simplex, furunculosis, wartsa | 1 |
| Extracutaneous infections | 18 |
| Oral candidiasis | 5 |
| Pneumonia | 3 |
| Sepsis | 2 |
| Herpetic keratitis, genital herpes, urinary infection, gastroenteritis, hepatitis C, bronchitis, CMV reactivation, upper airway infectiona | 1 |
| Noninfectious cutaneous complication | 10 |
| Striae | 3 |
| Rosacea | 2 |
| Monomorphous acne, erythema multiforme, decubitus ulcers, acute lipodermatosclerosis, hair lossa | 1 |
| Endocrinological complications | 29 |
| Cushing syndrome | 14 |
| Hyperglycemia/diabetes | 9 |
| Adrenal insufficiency | 5 |
| Secondary hyperparathyroidism | 1 |
| Hematologic | 8 |
| Anemia | 5 |
| Leukopenia | 2 |
| Monoclonal gammopathy | 1 |
| Bone/skeletal | 27 |
| Osteoporosis/osteopenia | 15 |
| Vertebral fractures, myalgia, muscle weaknessa | 5 |
| Crystal arthritis, arthralgiaa | 1 |
| Ophthalmological complications | 16 |
| Cataracts | 11 |
| Glaucoma | 4 |
| Xerophthalmia | 1 |
| Abnormal lipid metabolism | |
| High triglycerides and cholesterol | 11 |
| Psychiatric complications | 5 |
| Depression | 2 |
| Anxiety, anguish, psychosis | 1 |
| Digestive complications | 5 |
| Gastrointestinal bleeding | 3 |
| Diarrhea | 2 |
| Hepatic complications | |
| Elevation transaminases (noninfectious cause) | 1 |
| Cardiovascular complications | 16 |
| Hypertension | 5 |
| Deep vein thrombosis | 4 |
| Edema, heart failurea | 3 |
| Thromboembolism | 1 |
| Renal and urological complications | 7 |
| Proteinuria, kidney failurea | 2 |
| Hematuria, crystallization, acute urine retentiona | 1 |
| Disturbances in electrolyte metabolism | 3 |
| Hyperkalemia, hyponatremia, hypomagnesemiaa | 1 |
| Constitutional and general health complications | 11 |
| Weight loss | 4 |
| Weight gain | 3 |
| Asthenia, adynamiaa | 2 |
| Other | 6 |
| Amenorrhea, metrorrhagiaa | 2 |
| Gingival hypertrophy, tremora | 1 |
| Total | 190 |
PV presenting as mucosal lesions was more common in women (n=15 [79%]) than men (n=5 [39%]) (P=.03).
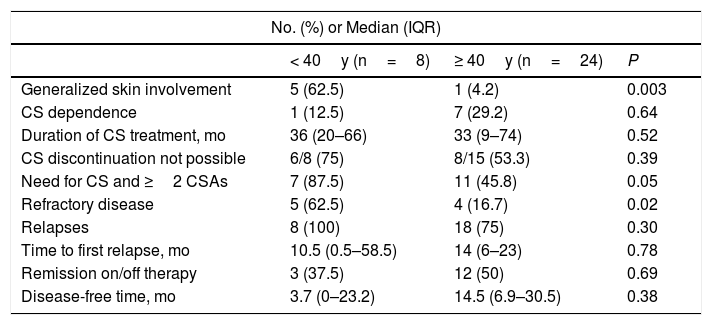
The distribution of study variables according to onset of PV before or after 40 years of age is shown in Table 6. Patients who developed PV before the age of 40 years were more likely to have generalized skin lesions affecting the face, scalp, trunk, and extremities (n=5 [63%], P=.003). They also required more than 2 treatments, including systemic corticosteroids and 1 or more corticosteroid-sparing agents (P=.05), experienced more relapses (P=.30), and were more likely to have refractory disease (P=.02). Patients who developed PV after the age of 40 years were more likely to experience disease remission on or off therapy (P=.69) and to have a longer disease-free period (on average, 11 months longer) (P=.38). These differences, however, were not statistically significant.
Differences Between Patients With Pemphigus Vulgaris by Age (n=32).
| No. (%) or Median (IQR) | |||
|---|---|---|---|
| < 40y (n=8) | ≥ 40y (n=24) | P | |
| Generalized skin involvement | 5 (62.5) | 1 (4.2) | 0.003 |
| CS dependence | 1 (12.5) | 7 (29.2) | 0.64 |
| Duration of CS treatment, mo | 36 (20–66) | 33 (9–74) | 0.52 |
| CS discontinuation not possible | 6/8 (75) | 8/15 (53.3) | 0.39 |
| Need for CS and ≥2 CSAs | 7 (87.5) | 11 (45.8) | 0.05 |
| Refractory disease | 5 (62.5) | 4 (16.7) | 0.02 |
| Relapses | 8 (100) | 18 (75) | 0.30 |
| Time to first relapse, mo | 10.5 (0.5–58.5) | 14 (6–23) | 0.78 |
| Remission on/off therapy | 3 (37.5) | 12 (50) | 0.69 |
| Disease-free time, mo | 3.7 (0–23.2) | 14.5 (6.9–30.5) | 0.38 |
Abbreviations: CS, corticosteroids; CSAs, corticosteroid-sparing agents; IQR, interquartile range.
Mucosal involvement as a presenting condition was an independent predictor of lesions at multiple mucosal sites (P=.003).
DiscussionOur study reveals 2 important findings. First, onset of PV before the age of 40 years may be a predictor of poor prognosis, since patients who developed PV at a younger age were more likely to have generalized skin lesions, require a higher number of corticosteroid-sparing agents, and have refractory disease. Second, patients with lesions that began in the mucosa were more likely to develop lesions at multiple mucosal sites, irrespective of age or sex.
The milder course of PV in patients with disease onset after the age of 40 years could be due to immunosenescence, similarly to in late-onset systemic lupus erythematosus. Immunosenescence predisposes older adults to a high prevalence of autoantibodies, autoimmune diseases, cancer, and infections. However, late-onset autoimmune diseases usually follow a milder clinical course and respond satisfactorily to immunosuppressive treatments due to the presence of more peripheral T regulatory cells.18,19
On comparing our results with those reported by Svecova20 for a series of patients with PV in Slovakia, we found a number of similarities in terms of sex (female predominance), frequency of mucosal involvement at onset and relapses, genital involvement, and use of azathioprine (main corticosteroid-sparing agent). Differences were observed for involvement of more than 1 mucous membrane (more common in the series described by Svecova [68%]) and for treatments. Svecova reported a higher starting dose of systemic corticosteroids (1.5–2mg/kg/d in 64% of patients) and a lower use of corticosteroid-sparing agents (29%). The author also reported fewer refractory cases (14%), a higher rate of remission on or off therapy (75%), and higher overall mortality (11%).
Treatment in our setting, which consisted of lower starting doses of systemic corticosteroids and a greater use of corticosteroid-sparing therapy, resulted in a similar relapse rate, a lower remission rate, and a higher rate of refractory disease. In light of the advances in our understanding of the usefulness of rituximab in PV and the expert recommendations on the diagnosis and management of this disease,12,13 clinical practice guidelines for Latin American countries will probably be modified. There is, however, still no consensus on the appropriate interval for maintenance therapy (6 months vs. 12 months) or on the optimal dose (500mg vs. 1g) for preventing relapse and maintaining PV in remission.21 Joly et al.12 proposed that the first maintenance cycle should be given after 6 months of treatment, because 73% of the patients in their study experienced relapse before 12 months. In our study, 4 patients experienced relapse after the first cycle of rituximab and 1 after the second cycle.
Although this is not the first series to be published on PV, we have described in detail 2 novel aspects that contribute to our understanding of this disease: the need for hospital admission among patients with PV and associations between age at disease onset and a range of variables.
The limitations of our study are inherent to its design (retrospective, single-center study with a small sample) and the use of different treatments during the study period. In addition, we did not have the facilities to test for anti-desmoglein autoantibodies. Finally, it was difficult to compare some of our findings with those of other studies due to the lack of global agreement on what constitutes mild, moderate, and severe PV.13 In our opinion, it is necessary to establish cutoff points to define disease severity and to standardize concepts such as corticosteroid dependence in PV.
In conclusion, patients with PV onset before the age of 40 years have a worse prognosis, and disease that begins as mucosal involvement is an independent predictor of subsequent involvement of multiple mucosal sites.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Cura MJ, Torre AC, Cueto Sarmiento KY, Bollea Garlatti ML, Riganti J, Puga MC, et al. Pénfigo vulgar: estudio de cohorte retrospectivo de sus características clínicas, tratamientos empleados y evolución. Actas Dermosifiliogr. 2020;111:398–407.