Pembrolizumab is a monoclonal antibody that belongs to a group of new antitumor drugs that stimulate the immune system. These drugs act by blocking key steps in the immune cascade. Specifically, pembrolizumab blocks the programmed cell death receptor (PD-1) whose function is to induce T-cell apoptosis, preventing excessive proliferation and function; this inhibition therefore leads to stimulation of the immune response.1 A number of cancers, including melanoma and lung, kidney, and breast cancer, can present overexpression of PD-1 ligand (PD-L1) by the tumor cells as a mechanism of immune evasion. Blockade of the PD-1 receptor would help to end this evasion.2,3
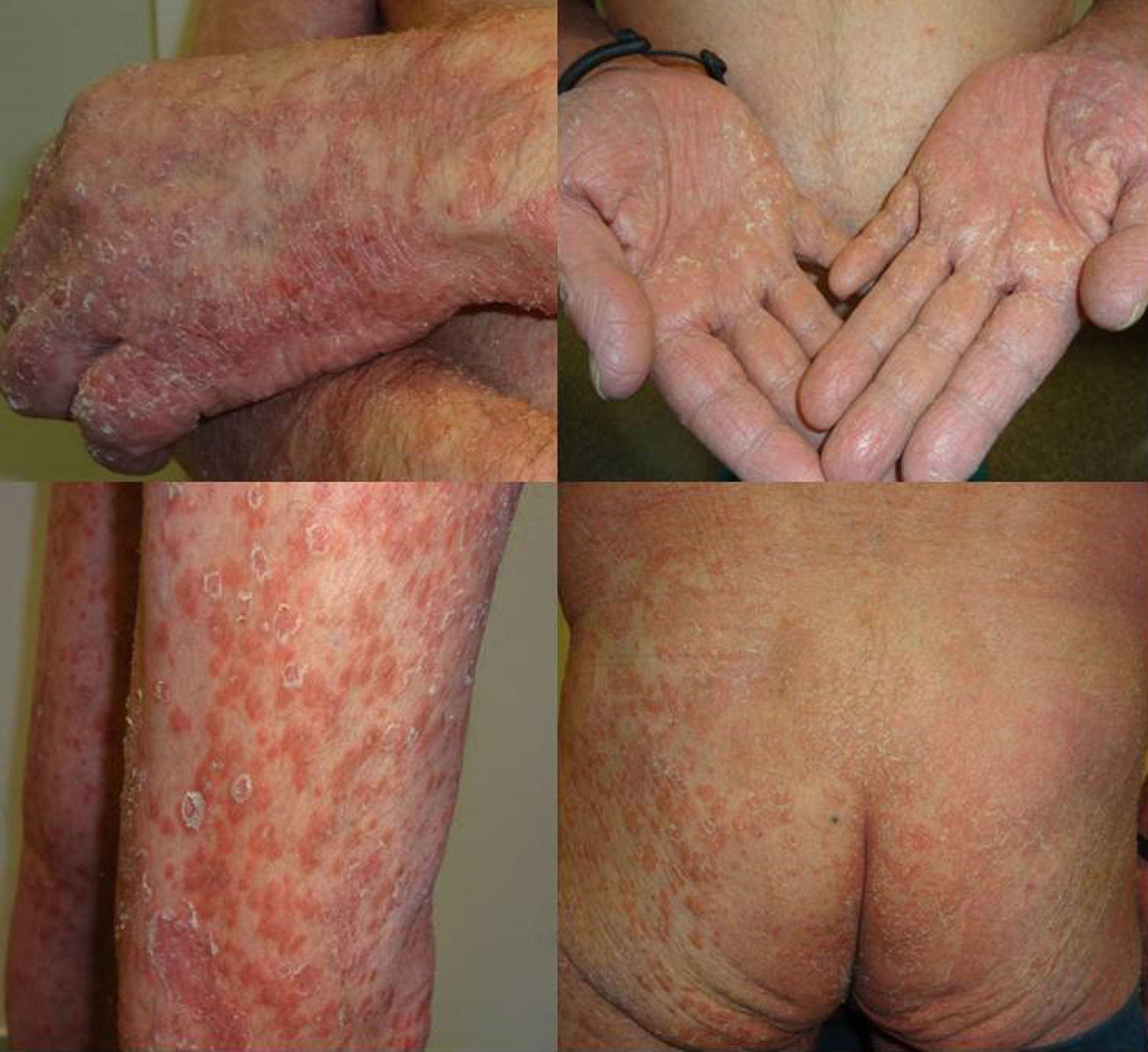
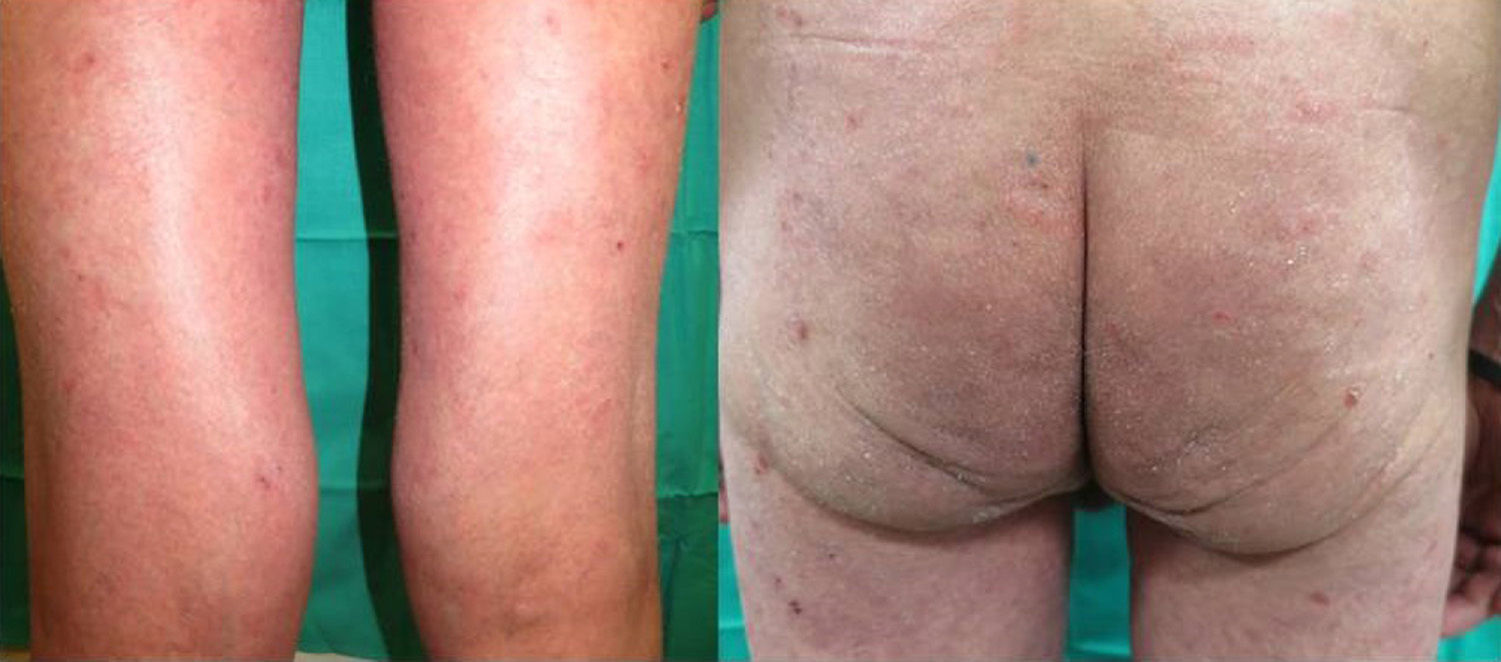
We present the case of a 67-year-old man with a history of myocardial infarction and cerebellar stroke, diagnosed 3 months earlier with metastatic adenocarcinoma of the lung with PD-L1 expression in more than 5% of tumor cells, measured immunohistochemically. The patient was referred to dermatology for evaluation and treatment of his psoriasis before entering a clinical trial investigating pembrolizumab for his lung neoplasm. The patient had psoriasis affecting the elbows, knees, and buttocks; the disease was stable on topical treatments, with a psoriasis area severity index (PASI) score of 3 and body surface area (BSA) involvement of 5%. The psoriasis deteriorated progressively after the first cycle of pembrolizumab, with a PASI score of 22 and BSA of 60%; after the second infusion, the severity became almost compatible with erythrodermic psoriasis, with a BSA of 81% and a PASI score of 33 (Fig. 1). Treatment was started with acitretin at a dose of 25mg/d. This had to be increased to 35mg/d after the deterioration seen during the second cycle. The tumor response to pembrolizumab was excellent after the second infusion of the drug, as was the improvement of his psoriasis with acitretin after 6 weeks of treatment. It was therefore decided to continue treatment with both drugs. There have been no further outbreaks of psoriasis during the following 3 infusion cycles. Three months after the initial outbreak, the patient had a PASI score of 4 (Fig. 2), which allowed us to reduce the dose of acitretin to 20mg/d.
Psoriasis is a chronic inflammatory disease. Evidence points ever more to an autoinflammatory disorder in which hyperreactive T cells produce large amounts of interleukins and tumor necrosis factor, leading to increased epidermal turnover. To date there are no reports of a deterioration or induction of psoriasis in patients treated with pembrolizumab or other immune checkpoint blockers, though, given the drug's mechanism of action and the pathophysiology of psoriasis, it would appear logical that such adverse reactions could occur. Recent studies have shown decreased expression of the PD-1 receptor in peripheral blood lymphocytes in patients with psoriatic arthritis and rheumatoid arthritis; this appears to show an inverse correlation with severity of the joint disease, as it would reduce the lymphocyte population susceptible to inhibition by the PD-1 pathway, although it does not seem to affect the PASI of patients with psoriasis.4,5 Based on this evidence, it may be surmised that certain quantitative changes take place in the PD-1+ T cells in patients with psoriasis, leading to T-cell hyperactivity that would increase exponentially with the use of PD-1–receptor blockers, explaining the notable deterioration in our patient's psoriasis. Confirmation of this hypothesis would require larger series of patients with psoriasis, and would need to include measurement of PD-1+ lymphocyte count by flow cytometry and immunohistochemistry techniques and of the PD-L1 levels by immunohistochemistry. In the way, we could define more accurately the pathophysiology of psoriasis and even open the door to new therapeutic targets.
There are still no publications on the treatment of psoriasis induced or exacerbated by immune checkpoint blockers. Based on our experience, acitretin could be a good option to achieve optimal control of a patient's psoriasis and would be compatible with continuation of the anticancer treatment. Studies of patient series are needed to confirm our clinical experience.
In general, the main side effects associated with the new immune checkpoint blockers are derived from their pharmaceutical action, provoking an autoimmune response against different body tissues. The most commonly described alterations have been hypo- and hyperthyroidism, hepatitis, diarrhea, enterocolitis, and hypophysitis. Skin manifestations include pruritus and a maculopapular rash, and there have even been reports of erythroderma, Stevens-Johnson syndrome, and toxic epidermal necrolysis. Patients at highest risk of developing these side effects are those with a history of autoimmune disease, which is a relative contraindication to the use of such drugs.6–8
The immune checkpoint blockers are achieving a good response in certain types of cancer.9 This would suggest that their use will increase in cancer patients. We must be alert to their adverse effects and perform more thorough follow-up and therapeutic management in patients with underlying autoimmune or autoinflammatory diseases.
We would like to thank Dr. Óscar Juan Vidal of the Oncology Department of Hospital Universitari i Politécnic La Fe in Valencia, Spain, for his help.
Please cite this article as: Sahuquillo-Torralba A, Ballester-Sánchez R, Pujol-Marco C, Botella-Estrada R. Pembrolizumab, un nuevo fármaco capaz de inducir un brote psoriasis. Actas Dermosifiliogr. 2016;107:264–266.