Atopic dermatitis is one of the most common skin disorders in children and it can negatively affect both children and their families. The purpose of this study was to investigate the effect of atopic dermatitis on quality of life related to maternal health and maternal obsessive compulsive symptoms.
MethodsA cross-sectional study was conducted in the pediatric and dermatology polyclinics. The SCORAD index was used for determining the severity of disease, and the Maudsley Obsessive Compulsive Inventory (MOCI) and SF-36 form were applied to the participants’ mothers.
ResultsA total of 120 children and their mothers participated the study. Comparing the atopic dermatitis group and the healthy control group, no statistically significant differences were seen in terms of MOCI and SF-36 scores, except for the physical functioning subscore.
ConclusionThe results showed that having a child with atopic dermatitis and the severity of the disease do not influence their mothers in terms of obsessive-compulsive symptoms and health-related quality of life, except for physical functioning scores.
La dermatitis atópica es uno de los trastornos cutáneos infantiles más comunes, que puede afectar de manera negativa tanto a los niños como a sus familiares. El objetivo del presente estudio era investigar el efecto de la dermatitis atópica en la calidad de vida relacionada con la salud materna y los síntomas obsesivo-compulsivos maternos.
MétodosSe dirigió un estudio transversal en policlínicos pediátricos y dermatológicos. El índice SCORAD de utilizó para determinar la gravedad de la enfermedad, y el inventario de obsesiones-compulsiones de Maudsley y el cuestionario SF-36 se entregaron a las madres de los participantes.
ResultadosEn el estudio participaron un total de 120 niños y sus madres. Al comparar el grupo con dermatitis atópica con el grupo de control no se encontraron diferencias con significación estadística respecto a las puntuaciones en el inventario de obsesiones-compulsiones de Maudsley y SF-36, salvo por la subpuntuación de la función física.
ConclusiónLos resultados demostraron que tener un hijo con dermatitis atópica y la gravedad de la enfermedad no influyen en las madres por lo que respecta a sus síntomas obsesivo-compulsivos ni su calidad de vida, salvo por las puntuaciones de función física.
Atopic dermatitis (AD), also known as atopic eczema, is a chronic, inflammatory, clinically defined disease predominantly affecting the skin that seriously disturbs the quality of life of these patients.1 AD is one of the most common skin disorders in children. It affects around 20% of the pediatric population and up to 3% of adults in western societies.2
The pathophysiology of AD is the result of a complex interaction between various susceptibility genes, host environments, infectious agents, defects in skin barrier function, and immunological responses.3
When caring for a child with eczema, the difficulties and time-consuming nature of managing complicated skin treatments compound the impact of sleep deprivation on the parents. Su et al. suggested that 2–3h per day are required to look after a child with eczema4 and parents may also lose time from work and suffer financial loss as a result of caring for their child.4–8 Practical problems of everyday care are also of great concern and include increased laundry, house cleaning and food preparation, shopping and house dust mite regimes.7 Lawson et al. found that over 90% of families reported problems with practical care, and this was flagged as one of the most problematic areas for them. Inevitably, there are lifestyle restrictions for the family as well as the child, both at home and socially. This may include limitations of family diet, eating out, pet ownership and avoidance of certain household products such as soaps and perfumed products. Difficulties of coping with the child outside the home environment may restrict family holiday choice and there are often problems with finding appropriate childcare or babysitters.7 Indeed the psychological distress and the practical difficulties of caring for a child with AD are the most frequent and problematic aspects from the parents’ perspective and often relate to the disease severity.9–12
Parents with a young child with AD reported experiencing significantly more parenting stress than a control group with a healthy child and described themselves as significantly more depressive, hopeless, anxious and overprotective.13
In many patients, atopic dermatitis takes a chronic, relapsing course where it is not possible to predict periods of activity or pinpoint aggravating factors. However, certain exposures are well known for aggravating eczema and should be avoided. Several infections, notably staphylococci, are frequent causes of exacerbations as are some foods, particularly in those cases where a patient is sensitized to any food. Lastly, many patients report that stressful living aggravates their eczema.14 Parents are very sensitive and anxious about aggravating factors and also parents have anxieties associated with corticosteroid treatment.15
Obsessive compulsive disorder (OCD) is a complex condition characterized by recurrent, intrusive, unwanted ideas, thoughts or impulses (obsessions) and attempts to reduce or neutralize the anxiety or prevent a dreaded outcome associated with the obsessions through carrying out repetitive ritualistic behavioral or mental actions (compulsions).16 OCS (obsessive compulsive symptom) is commonly associated with depression, anxiety, and marked impairment of professional and social functioning. Both aggravating factors and having to use steroids can lead to anxiety, especially including obsessive compulsive symptoms in parents.
The aims of this study were:
- 1)
To investigate the severity of atopic dermatitis in children and its effect on maternal quality of life.
- 2)
To investigate the magnitude of the effect of atopic dermatitis on maternal obsessive compulsive symptoms.
- 3)
To investigate the relationship between parental quality of life and maternal obsessive compulsive symptoms.
This study was a cross-sectional survey conducted at the pediatric and dermatology clinics of Turgut Ozal University between 15th September 2012 and 15th September 2013. All patients were evaluated by the same dermatologist (CG). We included consecutive children attending the pediatric and dermatology clinics who fulfilled the Hanfin and Rajka diagnostic criteria for eczema aged between one month and six years old. We recruited age and gender-matched healthy controls to compare the severity of parental OCS and Quality of Life with AD. These healthy controls were children attending for well child follow-up.
Mothers were the preferred caregiver for this study as they are considered to be the primary caregivers for children in Turkey.
The exclusion criteria for mothers were: the presence of chronic diseases, being single parent, and the use of neuropsychiatric medications considered likely to have a major impact on their psychological situation and quality of life.
QuestionnairesAssessment of disease severityThe SCORAD Index is the best validated scoring system for atopic dermatitis (AD). To measure the extent of AD, the rule of nines is applied on a front/back drawing of the patient's inflammatory lesions. The extent can be graded from 0 to 100. The intensity part of the SCORAD consists of six items: erythema, edema/population, excoriations, lichenification, oozing/crusts and dryness. Each item can be graded on a scale from zero to three. The subjective items include daily pruritus and sleeplessness. The SCORAD Index formula is: A/5+7B/2+C. In this formula, A is defined as the extent (0–100), B is defined as the intensity (0–18) and C is defined as the subjective symptoms (0–20). The maximal score of the SCORAD Index is 103. The objective SCORAD consists of the extent and the intensity items and the formula is A/5+7B/2. The maximal objective SCORAD score is 83 (with 10 additional points for severe disfiguring eczema of the face and hands).11–17
SCORAD was used because it is a useful and practical tool for the assessment of the severity of AD. In this study, the same observer assessed the disease severity in all cases, which added strength to the results, however, observer bias can never be totally excluded.
The patients were classified into the following three categories depending on the objective SCORAD scores: mild disease (SCORAD <20), moderate disease (SCORAD 20–40), and severe disease (SCORAD >40). Clinical examination and evaluation of disease severity were made by the same physician (CG).
Assessment of obsessive compulsive symptomsThe Maudsley Obsessive Compulsive Inventory (MOCI) is a 30-item questionnaire that evaluates OCS.18 It employs a dichotomous response format, and the total scores range from 0 to 30. We measured the MOCI total score and fear of contamination subscale score. The cutoff of the MOCI total score or subscales score has not been determined for Turkey.
Quality of life measurementSF-36 is a generic, self-administered Health-Related Quality of Life (HRQL) questionnaire, consisting of 36 items relating to eight domains, including physical function (ten items), physical role functioning (four items), emotional role functioning (three items), bodily pain (two items), social functioning (two items), mental health (five items), energy/vitality (four items), and general health perception (five items). Sub-inventories evaluate health from 0 to 100, with 0 indicating poor health and 100 indicating good health. The SF-36 is currently the most widespread generic HRQL instrument. The SF-36 has excellent reliability and construct and criterion validity.19 Turkish validity and reliability studies have also been performed.20
Statistical analysisStatistical analysis was conducted using SPSS for Windows (version 16.0) statistical software. Data for continuous variables are expressed as mean±SD values, and those for categorical variables are expressed as frequencies. Analysis of variance (ANOVA) was used to compare the SF-36 scores of atopic dermatitis patients by severity of atopic dermatitis, and Student's t-test was applied to compare variables between children with atopic dermatitis and healthy controls. Pearson correlation coefficients were used to determine the relationship between MOCI and SF-36 scores, and also between each of these scores and the SCORAD index. A p-value of <0.05 was considered statistically significant.
Approval for conducting this study was obtained from the research and ethical committee of Fatih University and written informed consent was obtained from all participating mothers.
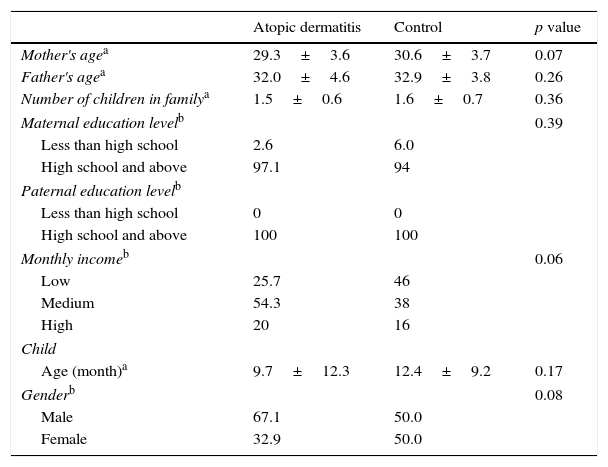
ResultsA total of 120 children and their mothers participated. Of these, 70 children had AD and 50 were considered as healthy controls. Comparison of demographic information for the children and their families is shown in Table 1. We did not find any statistical differences between the AD group and the healthy control group in terms of child age and gender, mothers’ age and education level, fathers’ age and education level, family income, or the number of children in the family (p>0.05).
Comparision of demographic characteristics of atopic dermatitis group and control group.
| Atopic dermatitis | Control | p value | |
|---|---|---|---|
| Mother's agea | 29.3±3.6 | 30.6±3.7 | 0.07 |
| Father's agea | 32.0±4.6 | 32.9±3.8 | 0.26 |
| Number of children in familya | 1.5±0.6 | 1.6±0.7 | 0.36 |
| Maternal education levelb | 0.39 | ||
| Less than high school | 2.6 | 6.0 | |
| High school and above | 97.1 | 94 | |
| Paternal education levelb | |||
| Less than high school | 0 | 0 | |
| High school and above | 100 | 100 | |
| Monthly incomeb | 0.06 | ||
| Low | 25.7 | 46 | |
| Medium | 54.3 | 38 | |
| High | 20 | 16 | |
| Child | |||
| Age (month)a | 9.7±12.3 | 12.4±9.2 | 0.17 |
| Genderb | 0.08 | ||
| Male | 67.1 | 50.0 | |
| Female | 32.9 | 50.0 | |
The median age of the children in the AD group was 1.5±0.6 and 74.3% of families were of medium or high socioeconomic status. The total mean (±SD) SCORAD score was 35.3±13.9 (7.4–66.6). Within the AD group 10 (14.3%) were mild, 32 (45.7%) moderate and 28 (40%) severe.
The mean duration of the complaints was 4.4±4.9 months in AD children. Only 17.1% of the children had an allergy test. More than half of children suffering from AD had a family history of allergy.
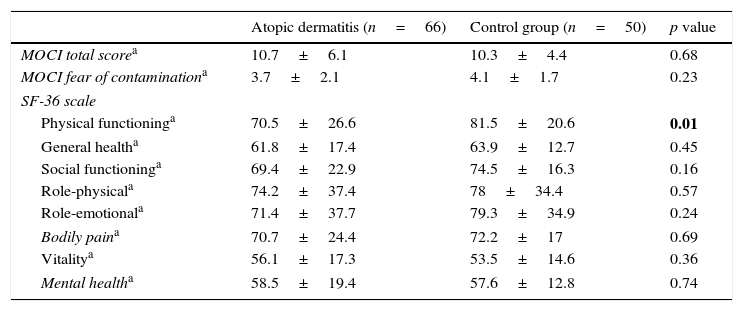
The comparison of the AD and control groups in terms of MOCI and SF-36 scores is detailed in Table 2.
Comparision of atopic dermatitis group and control group according MOCI and SF-36 scores.
| Atopic dermatitis (n=66) | Control group (n=50) | p value | |
|---|---|---|---|
| MOCI total scorea | 10.7±6.1 | 10.3±4.4 | 0.68 |
| MOCI fear of contaminationa | 3.7±2.1 | 4.1±1.7 | 0.23 |
| SF-36 scale | |||
| Physical functioninga | 70.5±26.6 | 81.5±20.6 | 0.01 |
| General healtha | 61.8±17.4 | 63.9±12.7 | 0.45 |
| Social functioninga | 69.4±22.9 | 74.5±16.3 | 0.16 |
| Role-physicala | 74.2±37.4 | 78±34.4 | 0.57 |
| Role-emotionala | 71.4±37.7 | 79.3±34.9 | 0.24 |
| Bodily paina | 70.7±24.4 | 72.2±17 | 0.69 |
| Vitalitya | 56.1±17.3 | 53.5±14.6 | 0.36 |
| Mental healtha | 58.5±19.4 | 57.6±12.8 | 0.74 |
MOCI: Maudsley Obsessive Compulsive Inventory.
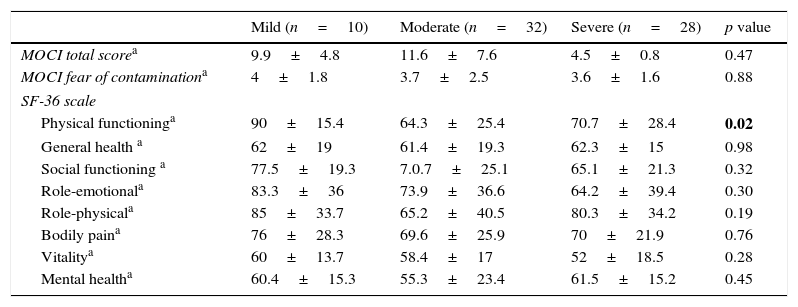
In analyzing the difference between the severity of AD in terms of MOCI and SF-36 scores, no influence was found except for SF-36 physical functioning. There was a significant difference between the mild and moderate groups (p=0.001). Mean scores and standard deviations on the MOCI and SF-36 depending on the severity of disease are shown in Table 3.
Mean scores and standard deviations on the MOCI and SF-36 depending on the severity of disease.
| Mild (n=10) | Moderate (n=32) | Severe (n=28) | p value | |
|---|---|---|---|---|
| MOCI total scorea | 9.9±4.8 | 11.6±7.6 | 4.5±0.8 | 0.47 |
| MOCI fear of contaminationa | 4±1.8 | 3.7±2.5 | 3.6±1.6 | 0.88 |
| SF-36 scale | ||||
| Physical functioninga | 90±15.4 | 64.3±25.4 | 70.7±28.4 | 0.02 |
| General health a | 62±19 | 61.4±19.3 | 62.3±15 | 0.98 |
| Social functioning a | 77.5±19.3 | 7.0.7±25.1 | 65.1±21.3 | 0.32 |
| Role-emotionala | 83.3±36 | 73.9±36.6 | 64.2±39.4 | 0.30 |
| Role-physicala | 85±33.7 | 65.2±40.5 | 80.3±34.2 | 0.19 |
| Bodily paina | 76±28.3 | 69.6±25.9 | 70±21.9 | 0.76 |
| Vitalitya | 60±13.7 | 58.4±17 | 52±18.5 | 0.28 |
| Mental healtha | 60.4±15.3 | 55.3±23.4 | 61.5±15.2 | 0.45 |
One way ANOVA.
Having a family history of allergies did not influence the MOCI and SF-36 scores (p>0.05).
There was no found correlation between SCORAD, MOCI and SF-36 scores. However, there was a low correlation between the MOCI total scores and SF-36 general health (p=0.01, r=−0.22), SF-36 physical functioning (p=0.02, r=−0.21), SF-36 social functioning scores (p=0.001, r=−0.35), SF-36 role-physical (p=0.03, r=−0.19), and SF-36 pain (p=0.001, r=−0.39) within the AD group. There was no correlation between the MOCI total scores and the SF-36 mental health, vital and emotional scores.
DiscussionThis hospital-based cross-sectional study did not support the hypothesis that parents of children with AD experience any decrement in their quality of life or have obsessive compulsive symptoms after comparison with a healthy control group, except for physical functioning subscores. To our knowledge there is not other study that examines maternal OCS for AD.
The results are in accordance with a study by Kano et al., who considered whether the OCS associated with Taurette syndrome (TS) and autism spectrum disorders (ASD) were also found in the parents of TS and ASD probands by comparing them with normal controls and no significant difference was found.21
Nearly 75% of families were of medium or high socioeconomic status and most had one child. The parents were knowledgeable about the disease and caring for a child with AD did not influence them negatively.
No correlation was found between having a family history of allergy and MOCI scores and subscores. When parents also suffered from allergic diseases, they were already experienced with the disease and its treatment and management, and were less anxious.
Ohya et al. found that the strongest predictor of adherence to therapy was good doctor-parent relationship, which are more important than maternal anxiety about using topical corticosteroids.22 In this study, the mothers of children with AD had attended a private hospital indicating that more time and attention had been applied. On the grounds that parents should have a good relationship with the doctor and should be informed in detail about the disease and its care, a decrease in parental quality of life and anxiety levels was found to be less than in the literature.7,23
In the SF-36, the only statistical difference between AD and controls was in the physical functioning scores. A decrease of health-related quality of parental life is well documented in the literature using SF-36 and other quality of life questionnaires.5,7,12
However, other studies used different instruments for determining health related quality of life, making any direct comparison not possible. While the severity of the disease relates to the quality of parental life in the literature;12 a statistically significant difference was only determined in the physical functioning scores.
A negative weak correlation was observed between the MOCI and SF-36 subscores. When the parents had obsessive compulsive symptoms, a decrease in the quality of life was encountered.
This study has some limitations. First, the study was based on a parental filled out questionnaire. A one-to-one psychiatric evaluation of participating mothers could have given better results. Second, the study population was small and all the parents had medium and high socioeconomic levels. A further study with larger groups from all socioeconomic status levels and including psychiatric evaluation should be carried out.
To our knowledge, the present study is the first to investigate the association between parental OCS and quality of life in AD children. The results showed that having a child with atopic dermatitis and also the severity of the disease do not influence mothers in terms of obsessive-compulsive symptoms and health related quality of life, except for physical functioning scores, among medium-high socioeconomic Turkish parents.
Ethical disclosuresProtection of human and animal subjectsThe authors state that the procedures followed in the research are in accor dance with the ethical guidelines of the responsible committee on human and animal research (institutional or regional) and in accordance with the World Medical Association and Declaration of Helsinki.
Confidentiality of dataThe authors state that the rights of their patients to privacy and confidentiality have been assured in accordance with that described in the section corresponding to these guidelines and that the article has avoided any type of identifying data in the text or images and, in any case.
Right to privacy and informed consentThe authors state that they have the informed consent of the patients for the participation in the study and publication of the results in Actas Dermo-Sifilográficas in the print version and Electronic (internet) and that this has been thus declared in the EES.
Conflicts of interestNo conflict of interest was declared by the authors.