Axillary hyperhidrosis (AH) and bromhidrosis are common causes of consultation in dermatology. Currently, the most widely prescribed treatment for AH is botulinum toxin, a very effective but temporary option; it is totally ineffective in bromhidrosis. Sympathectomy is an increasingly infrequent choice of treatment due to the high incidence of compensatory hyperhidrosis. We describe the treatment of AH and bromhidrosis with a novel microwave device that can fibrose eccrine and apocrine glands, achieving possibly permanent results. The procedure should preferably be performed under tumescent anesthesia. Side effects, principally local inflammation, are transient. Clinical effectiveness and safety, supported by recently published studies, position this technique as a first-choice option both for hyperhidrosis and for bromhidrosis.
La hiperhidrosis axilar (HA) y la bromhidrosis son un motivo de consulta frecuente en dermatología. Hoy en día el tratamiento más indicado es la inyección de toxina botulínica, una opción muy eficaz pero con el inconveniente de su temporalidad, y en el caso de la bromhidrosis su nula eficacia. Por otra parte, la indicación de la simpatectomía cada vez se recomienda menos por la alta incidencia de hiperhidrosis compensatoria.
En este artículo se expone el tratamiento de la HA y la bromhidrosis con un dispositivo novedoso de microondas, capaz de reemplazar las glándulas ecrinas y apocrinas por fibrosis, consiguiendo unos resultados posiblemente permanentes. El procedimiento debe realizarse preferiblemente con anestesia local tumescente. Los efectos secundarios son temporales, principalmente inflamación local. Su eficacia clínica y seguridad sitúan a esta técnica, avalada por estudios recientes publicados, como una alternativa de primera elección tanto para la HA como para la bromhidrosis.
Axillary hyperhidrosis (AH) is a common complaint in the general population, one that leaves a psychological burden on those who experience it. The condition is defined as sweating excessively–more than necessary for physiological requirements–regardless of the ambient temperature. The prevalence in the United States has been reported to be 1.4%,1 but between 20% and 33% of persons with AH report that their activities of daily living are affected.2,3
Treatment has evolved a great deal in recent years, and there are now effective, long-lasting remedies. We provide a succinct review of the traditional treatments available and focus on a novel modality, microwave therapy, which will undoubtedly earn a place among the alternatives on offer. Microwave therapy has also proven effective in bromhidrosis, defined as an unpleasant odor associated with sweating.
Traditional TreatmentsVarious drugs have been used to block AH over the course of time with varying degrees of success. Side effects have placed limits on their use. One of the most often prescribed is oxybutynin (5–15mg/d) because of its good safety profile and long-term clinical effectiveness.4 Randomized placebo-controlled trials have shown this drug to be effective, although dry mouth is a common adverse event, reported in 43% of users.5 Clonidine, another drug with which there is clinical experience,6 is available in both oral and topical forms. A recent review summarized all medical treatment options for AH.7
Botulinum toxin A (BTX-A) is a modern classic that has been used to treat AH since 1996.8 Although highly effective, achieving nearly complete anhidrosis, the main problems of BTX-A injections are that their effect is temporary and treatment is costly. Some studies have shown the effect is longer lasting if injections are repeated over time. One group found that the duration of effect increased from 4 to 4.5 months after the second injection and to 5 months after the third.9 Another reported that the 5.5-month duration of the first treatment's effect increased to 8.5 months after the last session.10
Various surgical techniques to remove eccrine glands have also been used, specifically Shelley's procedure,11 Breach's procedure, and liposuction with curettage. All these approaches lead to satisfactory but temporary results, and numerous complications have been reported. In terms of efficacy, for example, liposuction followed by curettage reduced sweating by 85% a month after the procedure, but that efficacy rate had fallen to 61% 2 years later.12
Finally, sympathectomy is probably the last therapeutic alternative offered to patients. It is prescribed when other options have failed. Although it is effective and provides permanent relief, compensatory hyperhidrosis and other potentially serious complications have been reported in up to 91% of patients.13
Microwave TherapyPrinciples and Mechanism of ActionMicrowaves have radiofrequencies within the nonionizing electromagnetic spectrum, which includes infrared waves and longer radio waves. Microwaves have the ability to heat substances through a process termed diathermy, which has been used for decades to coagulate tissue during surgery. These waves are relatively selective, preferably heating tissues with high water content, such as glands, by taking advantage of the greater dipolarity of water molecules compared to fat. This property proved useful in AH by enabling the design of a device to penetrate to the deep layer of subcutaneous dermal tissue with wavelengths of 5.8GHz. At that level (2 and 5mm deep), eccrine and apocrine glands can be destroyed through thermolysis. Histology shows that they are then replaced by fibrosis.14 The device (miraDry; Miramar Labs, Sunnyvale, CA, USA) protects the dermis and epidermis by a combined system of suction and cooling of the skin. Suction facilitates uniform treatment and prevents surrounding tissues (e.g., nerve endings in the brachial plexus) from being harmed. Two reviews by Jacob2 and Johnson et al.15 treat these principles and mechanisms of action in greater detail.
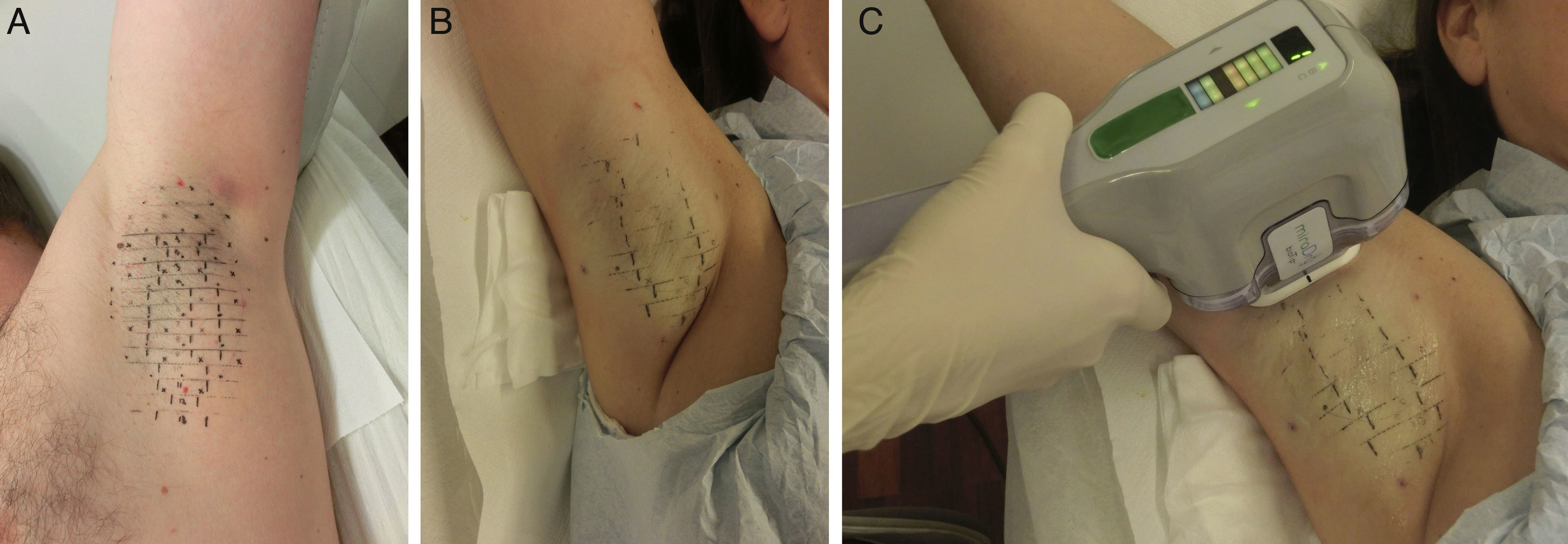
ProcedureThe procedure can be summarized in 3 steps. First a template that is sized to match the surface of the axillary vault is placed on the skin to guide marking landmarks, which show where the device's handpiece will be placed in a later step (Fig. 1A). Second, tumescent anesthesia is applied to the area (Fig. 1B). The actual treatment is given in the third step, in which the tip of the handpiece is applied to release microwave energy at the previously marked points (Fig. 1C). The device has different treatment settings. The program automatically used for the upper part of the axilla, where the brachial plexus is close to the surface, is the lowest setting (level 1) to safeguard against accidental injury. The remaining area of the axilla can be treated at the maximum setting (level 5), especially if tumescent anesthesia has been provided. An area 10×30mm is treated each time the handpiece is applied. Thus, depending on the size of the axillary vault, between 12 and 39 applications will be made. Depending on what treatment level is used (higher levels require a longer session), the procedure lasts between 25 and 40minutes per axilla.
The procedure is painful so local anesthesia must be provided. The templates also include points showing where the anesthetic should be injected. The number of injections ranges from 26 to 60 on each side, depending on the size of the axillary vault. Such a large number of infiltrations is also painful, even if an insulin or mesotherapy needle is used. Tumescent anesthesia is therefore preferred today. This safe technique is also applied in other clinical settings, including AH treated by curettage.12 A high volume–between 80 and 120mL, depending on the size of the vault–of a mixture of epinephrine and a 1% lidocaine solution in physiological saline is injected into each axilla. The procedure to provide tumescent anesthesia is practically painless and allows higher levels of microwave energy to be applied, making for greater efficacy. In addition, brachial plexus injury is less likely because the high volume of saline and anesthetic insulates the tissues. Finally, this technique uses less anesthetic than the initially recommended local anesthetic procedure.
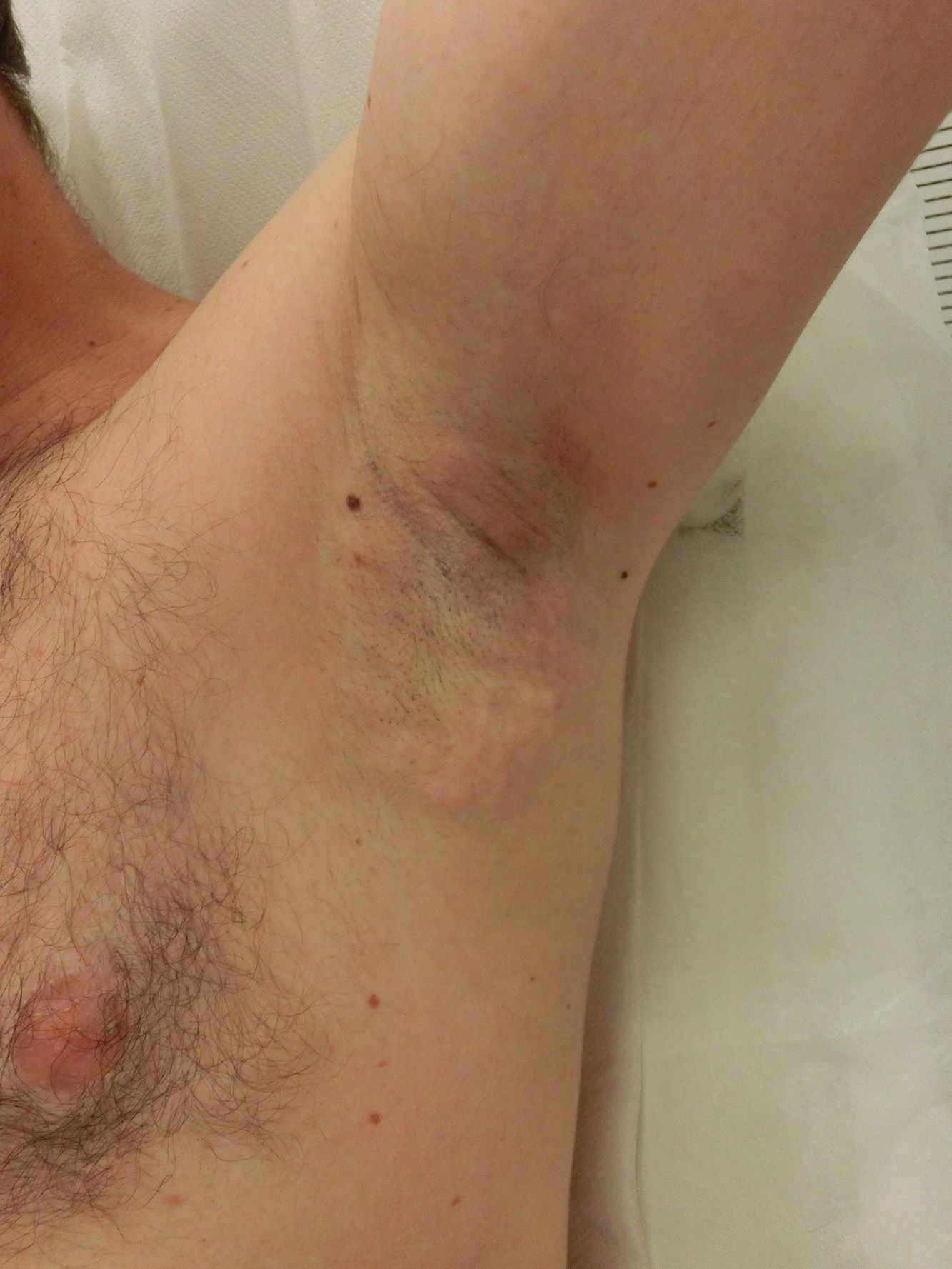
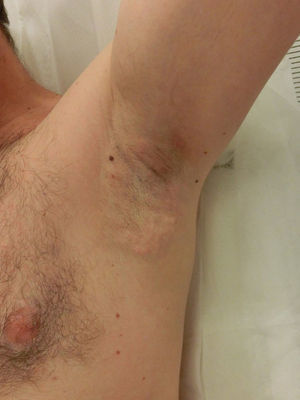
Side EffectsNearly all patients experience painful local inflammation and develop subcutaneous nodules (Fig. 2) that gradually disappear over the course of the first week. Also often observed is local bruising, a result of the suction applied. Oral anti-inflammatory medication is therefore routinely prescribed for the first 72hours, along with application of a cold pack. Discomfort can sometimes last several weeks or occasionally as long as 3 to 4 months, but without interfering with daily activities.
The only important complication described in the literature is neuropathy, reported in 2 cases. One patient had medial and cubital nerve damage that resolved fully by 12 months with rehabilitation.16 Paradoxically, that patient had been treated at the lowest energy setting and had received tumescent anesthesia, under optimal procedural conditions that usually safeguard against nerve damage. The other patient had neuropathy associated with muscle weakness, a clinical picture that resolved in 6 months.17
Compensatory hyperhidrosis seems to be a rare complication. It was reported in 2 patients, one of whom presented persistent facial sweating after the procedure.3 Finally, another possible complication is permanent hair loss, an effect that has been described in 26% of patients.17 This side effect has led to current interest in studying microwave therapy as a novel means of hair removal, especially for gray hair.
Clinical Trials and Case SeriesA few trials and case series have provided information on this newly available modality.3,14,17,18 Noteworthy was the randomized multicenter trial of Glaser et al.,3 who compared microwave treatments to sham treatments in 120 patients. Follow-up lasted 12 months in the treatment group of 81 patients. All enrolled individuals had scores of 3 or 4 on the Hyperhidrosis Disease Severity Scale (HDSS) (Table 1). Statistically significant improvement was observed 1 or 2 months after treatment in the active treatment group, where disease severity decreased in 89% of the patients (vs 54% in the sham-treatment group, P<.001). The effect difference was maintained at the 6-month follow-up. When the outcome measure of efficacy was a reduction of 2 or more points on the HDSS scale, 67% of patients in the active treatment group achieved success (vs 13% in the sham-treatment group). A similar degree of efficacy was maintained in the microwave-treated group at 12 months.
Hyperhidrosis Disease Severity Scale.
| 1: My sweating is never noticeable and never interferes with my daily activities. |
| 2: My sweating is tolerable but sometimes interferes with my daily activities. |
| 3: My sweating is barely tolerable and frequently interferes with my daily activities. |
| 4: My sweating is intolerable and always interferes with my daily activities. |
Ninety percent in a series of 31 patients showed improvement 12 months after microwave treatment (reaching HDSS scores of 1 or 2 from baseline scores of 3 or 4).17 Patient satisfaction with treatment was high in 90%. Finally, 83% of a series of 11 patients with both AH and bromhidrosis reported HDSS scores that were 2 points lower 7 months after treatment.14
Treatment of BromhidrosisBromhidrosis–a problem that can appear along with AH or in isolation–is another promising new indication for this therapy because microwaves are able to eliminate apocrine glands. A substantial number of patients in the series analyzed by Hong et al.17 mentioned they perceived no unpleasant body odor after treatments. Similarly, 93.8% of the patients in the series of Lee et al.14 reported a good or excellent response of body odor to microwave therapy. Only certain surgical techniques19 or certain laser therapies (e.g., interstitial Nd:YAG 1444nm treatments20 have been able to offer a degree of relief to patients with this problem to date.
Cost ConsiderationsCost is one of the most important problems of microwave therapy mainly because of the price of consumables and the time invested by staff who apply the treatments. No cost comparisons have been made between this modality and others, but we can calculate that 2 microwave sessions would cost as much as 6 BTX-A treatments priced around €500 per session. BTX-A has a mean duration of effect of 8 months. Therefore, the investment in microwave treatment will have paid for itself in about 4 years.
Our Clinical ExperienceOur experience using microwave treatments for AH has been very positive. We have treated 102 patients since the device became available in Spain in May 2015. Two sessions are usually scheduled 3 months apart, but we find that patients with moderate AH or bromhidrosis alone may only require a single session. Twenty percent of our first 50 patients continued to report excessive sweating after 2 treatment sessions at the initially recommended middle energy level and conventional local (not tumescent) anesthesia. The remaining 80% reported more than a 50% reduction in sweating. Our recent figures have been even better since we changed the protocol, using tumescent anesthesia and treating all patients at the highest energy level. Since that change, only 5% have failed to respond to treatment or reported insufficient improvement after 2 sessions. These results are similar to those published by other authors. Hong et al.,17 for example, found it necessary to use 3 sessions for 15 of their 31 patients (48%). Glaser and Coleman3 applied 3 treatments in 7 of their 81 patients (8%).
The permanent, or longer-term effect, of this modality in reducing AH remains to be demonstrated. The longest follow-up described to date was 2 years, during which the effect was maintained.18 Because axillary glands originate in embryonic tissue, they do not regenerate once they have been eliminated: therefore, it is reasonable to assume the improvement will not disappear over time and that we will be able to speak of a permanent outcome in the future. We are currently studying this question by means of periodic annual surveys of our patients to update their HDSS scores.
None of our 102 treated patients have reported serious or permanent side effects 16 months after treatment. Cases of compensatory hyperhidrosis after microwave treatment have not been reported in the literature. Five of our 102 patients did report having this problem to a mild degree after they were specifically asked about it, however. Locations were far from the axillas (e.g., the back, face, or upper part of the chest).
Finally, we wish to emphasize the high level of efficacy of this treatment in bromhidrosis when it was an isolated complaint (2 cases) as well as when associated with AH (13 cases). Microwave therapy nearly completely eradicated the problem for these patients.
ConclusionsGuidelines on AH currently specify the various types of surgical procedures that comprise the options available once medical treatments have failed to resolve the problem. We can say that this recommendation is out of date today. The development of new noninvasive alternatives such as microwave therapy should be considered before surgery is offered and in many cases can be suggested as an alternative to BTX-A injections. AH patients express a high level of satisfaction with microwave therapy. They evaluate it as superior to other options mainly because of its long-term effect. Although the results published thus far are very promising, longer-term studies are necessary before we can assume the benefits are permanent.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Sánchez-Carpintero I, Martín-Gorgojo A, Ruiz-Rodríguez R. Tratamiento con microondas en la hiperhidrosis y bromhidrosis axilar. Actas Dermosifiliogr. 2017;108:418–422.